Monoclonal Antibody Prevents Malaria In U S Adults Nih Trial Shows
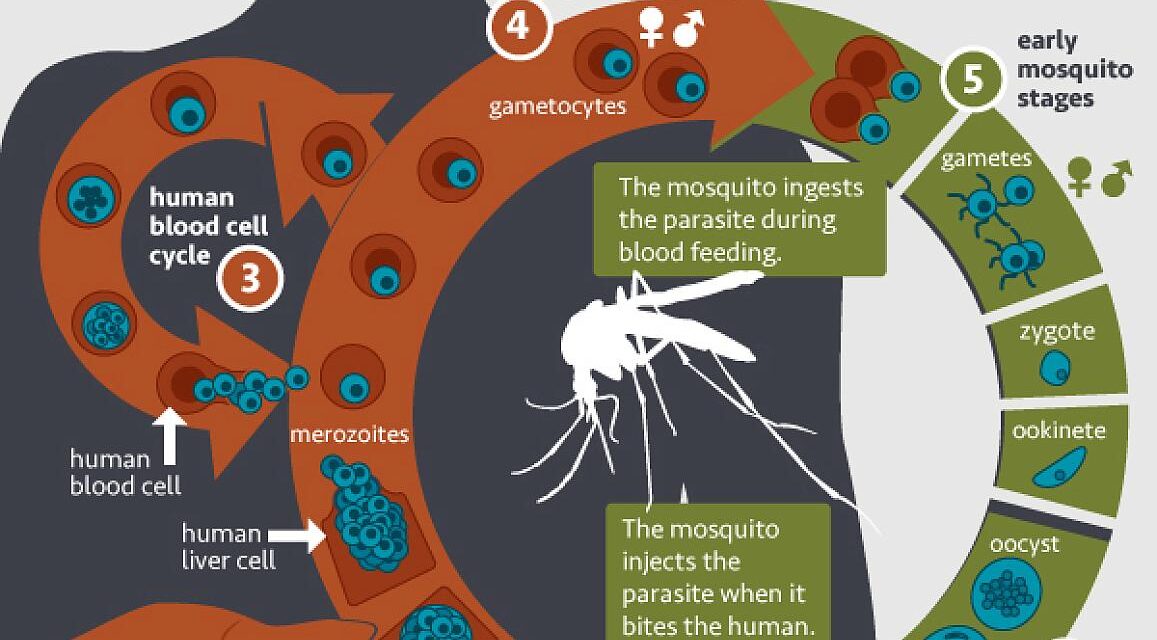
Monoclonal Antibody Prevents Malaria In U S Adults Nih Trial Shows One injection of a candidate monoclonal antibody (mab) known as l9ls was found to be safe and highly protective in u.s. adults exposed to the malaria parasite, according to results from a national institutes of health phase 1 clinical trial published in the new england journal of medicine. A monoclonal antibody prevented malaria in people for up to 36 weeks in a phase 1 clinical trial. such antibodies could have a variety of uses for travelers, military personnel, health care workers, and campaigns to control and eliminate malaria. colorized electron micrograph showing malaria parasite (right, blue) attaching to a human red blood.

First Human Trial Of Monoclonal Antibody To Prevent Malaria Opens Scientists from nih’s vaccine research center set out to develop a laboratory made antibody, called a monoclonal antibody, to neutralize the disease causing parasite before it can infect the liver and take hold in the body. they created a monoclonal antibody from the blood of a volunteer who received an experimental malaria vaccine. One injection of a candidate monoclonal antibody (mab) known as l9ls was found to be safe and highly protective in u.s. adults exposed to the malaria parasite, according to new results. additional. A phase 1 clinical trial testing the safety and effectiveness of a monoclonal antibody (mab) against malaria has begun enrolling healthy adult volunteers at the national institutes of health clinical center in bethesda, maryland. the trial, sponsored by nih’s national institute of allergy and infectious diseases (niaid), is the first to test mab cis43ls in humans. it aims to enroll up to 73. The limitations of this trial include its small size and the safety and efficacy data that were restricted to healthy adults in the united states who had never had malaria or received a vaccine.

Comments are closed.