Molecular Shapes Vsepr Theory Vrogue Co

Molecular Shapes Vsepr Theory Vrogue Co Because a lone pair is not shared by two nuclei, it occupies more space near the central atom than a bonding pair (figure 10.2.4 10.2. 4). thus bonding pairs and lone pairs repel each other electrostatically in the order bp–bp < lp–bp < lp–lp. in so 2, we have one bp–bp interaction and two lp–bp interactions. 4. The valence shell electron pair repulsion (vsepr) theory is a simple and useful way to predict and rationalize the shapes of molecules. the theory is based on the idea of minimizing the electrostatic repulsion between electron pairs, as first proposed by sidgwick and powell in 1940, [9] then generalized by gillespie and nyholm in 1957, [10] and.
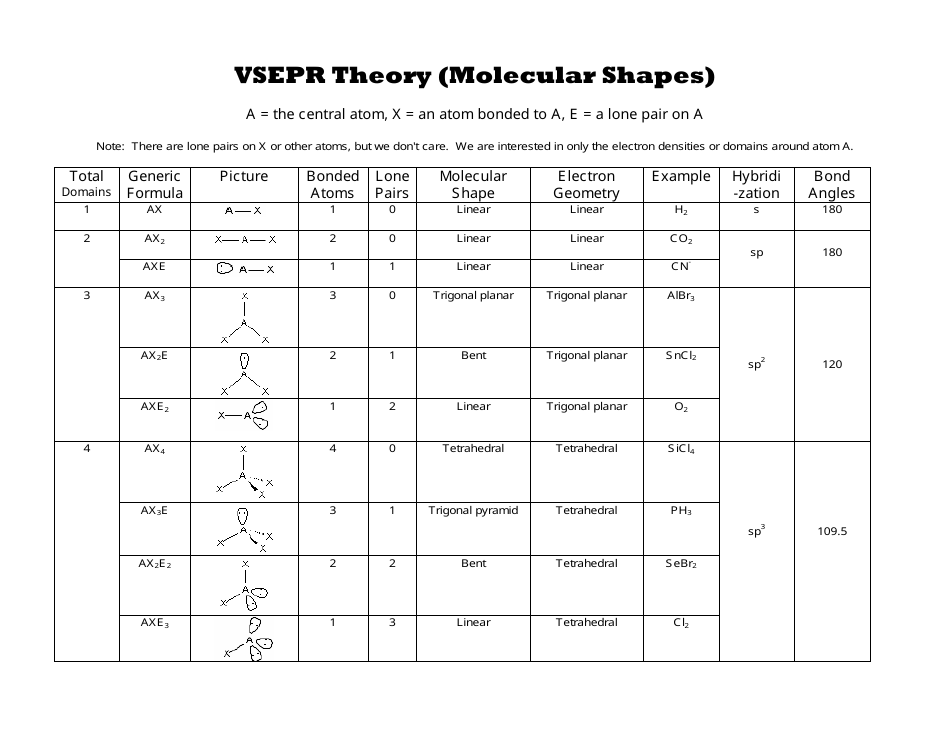
Vsepr Theory Geometry Of Organic Molecules Chemistry Vrogue Co When the two electron groups are 180° apart, the atoms attached to those electron groups are also 180° apart, so the overall molecular shape is linear. examples include beh 2 and co 2: figure 4.13.1 4.13. 1 beryllium hydride and carbon dioxide bonding. the two molecules, shown in the figure below in a "ball and stick" model. Explore molecule shapes by building molecules in 3d! how does molecule shape change with different numbers of bonds and electron pairs? find out by adding single, double or triple bonds and lone pairs to the central atom. then, compare the model to real molecules!. According to vsepr theory, a molecule is designated by the letters ax m e n. “a” represents the central atom, “x” represents the bonded atoms, “e” represents the lone pairs on the central atom, “m” is the number of electron groups or domains, and “n” is the number of lone pairs on the central atom. example: the water (h 2 o. Vsepr theory is short for valence shell electron pair repulsion theory, a method of organizing molecules based on their geometric structures. in chemistry, vsepr theory is based on the principle that each atom in a molecule will seek a geometry that maximizes the distance between valence electron pairs, thus minimizing electron electron.
Molecular Shapes Vsepr Theory Vrogue Co According to vsepr theory, a molecule is designated by the letters ax m e n. “a” represents the central atom, “x” represents the bonded atoms, “e” represents the lone pairs on the central atom, “m” is the number of electron groups or domains, and “n” is the number of lone pairs on the central atom. example: the water (h 2 o. Vsepr theory is short for valence shell electron pair repulsion theory, a method of organizing molecules based on their geometric structures. in chemistry, vsepr theory is based on the principle that each atom in a molecule will seek a geometry that maximizes the distance between valence electron pairs, thus minimizing electron electron. The valence shell electron pair repulsion (vsepr) theory is a model used to predict 3 d molecular geometry based on the number of valence shell electron bond pairs among the atoms in a molecule or ion. this model assumes that electron pairs will arrange themselves to minimize repulsion effects from one another. The bef 2 molecule adopts a linear structure in which the two bonds are as far apart as possible, on opposite sides of the be atom. as a simple example of vsepr theory, let us predict the structure of a gaseous bef2 bef 2 molecule. the lewis structure of bef2 bef 2 (figure 7.1.2) shows only two electron pairs around the central beryllium atom.

Main Vsepr Theory Molecular Shapes Chart Vsepr Theory Vrogueо The valence shell electron pair repulsion (vsepr) theory is a model used to predict 3 d molecular geometry based on the number of valence shell electron bond pairs among the atoms in a molecule or ion. this model assumes that electron pairs will arrange themselves to minimize repulsion effects from one another. The bef 2 molecule adopts a linear structure in which the two bonds are as far apart as possible, on opposite sides of the be atom. as a simple example of vsepr theory, let us predict the structure of a gaseous bef2 bef 2 molecule. the lewis structure of bef2 bef 2 (figure 7.1.2) shows only two electron pairs around the central beryllium atom.

Comments are closed.