Mole Conversions Chem Worksheets 11 3
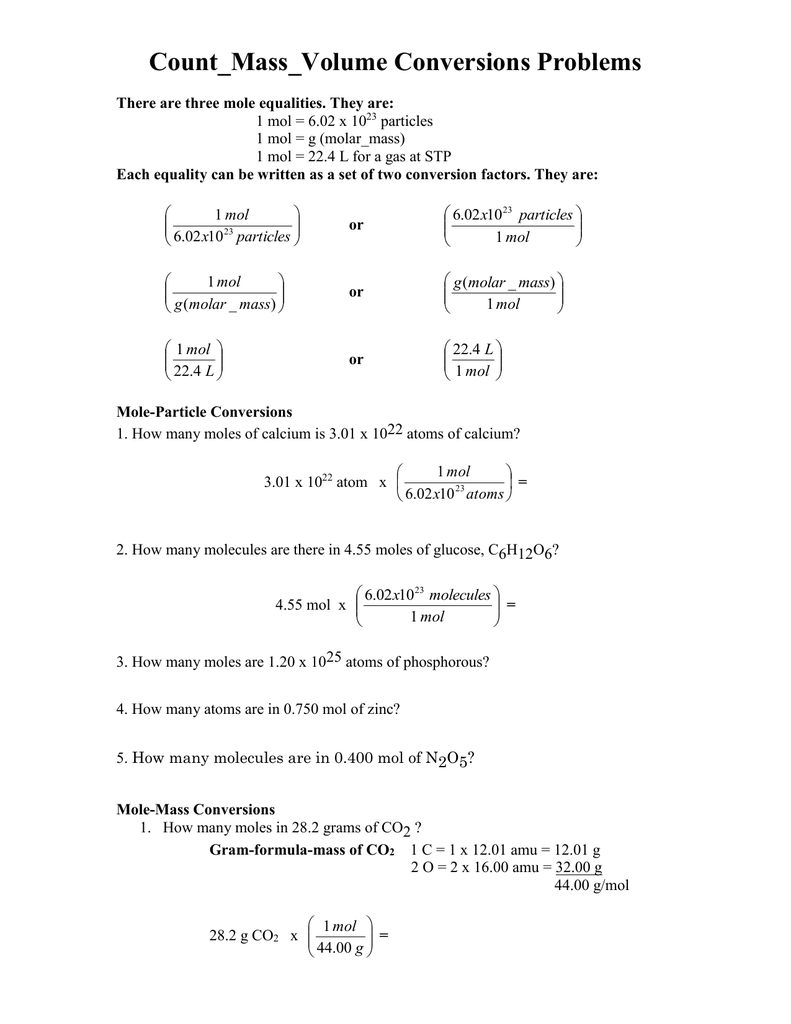
Mole Conversions Chem Worksheet 11 3 Mole conversions name chem worksheet 11 3. it is important to be able to convert units from and into units of moles. the mole is a unit for count, as is the dozen. a dozen is 12 items, but a mole is 602 000 000 000 000 000 000 000 , or 6.02 × 1023 particles. using the periodic table we can find the molar mass, or the mass of a. Mole worksheet #2 make the following conversions using unit analysis. use a separate piece of paper, show all work, and circle your final answer. (attach this sheet to your work). set a: one step problems: convert to moles: convert to mass in grams: 10.0 moles na 11. 12. 2.20 moles sn 13. 5.00 moles ag 14. 3.0 x 104 moles au 15. 1.00 x 10 7 moles b.

Mole Conversions Chem Worksheet 11 3 All assignments, notes & worksheets are to be submitted with the unit notebook. quiz corrections*: 1 2 mark for each correction. must be submitted with unit notebook. *completed on a separate page, questions written out in pen in full, explain why it was incorrect, explain how you corrected your mistake provide the correct answer in pencil. 2. what is the mass of 5 moles of fe2o3 ? 3. find the number of moles of argon in 452 g of argon. 4. find the grams in 1.26 x 10 4 mol of hc2h3o2. 5. find the mass in 2.6 mol of lithium bromide. mole volume conversions 1. determine the volume, in liters, occupied by 0.030 moles of a gas at stp. 2. how many moles of argon atoms are present in 11. How many moles of magnesium is 3.01 x 10²² atoms of magnesium? 3.01 x 10²² ato x (1mol 6.02 x 10²³ato) = 5 x 10⁻² ato mg how many molecules are there in 4.00 moles of glucose, c₆h₁₂o₆?. 5) mgcl2. 6) (nh4)2so4. there are three definitions (equalities) of mole. they are: 1 mole = 6.02 x 1023 particles. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp (you do not need to worry about this yet) each definition can be written as a set of two conversion factors.

Comments are closed.