Mole Concept Definition Formula Examples And Faqs
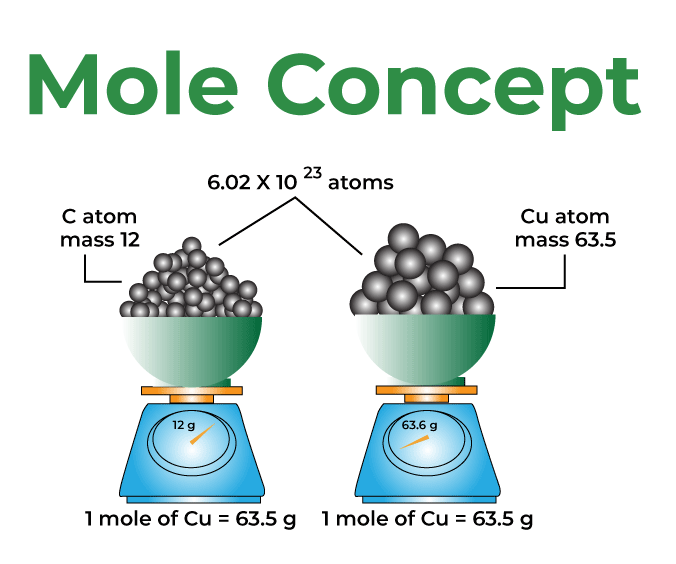
Mole Concept Definition Formula Examples And Faqs Mole concept is known as the method used to express the amount of substance. a mole is defined as the amount of substance containing the same number of different entities (such as atoms, ions, and molecules) as the number of atoms in a sample of pure 12c weighing precisely 12 g. even a gram of any pure element contains a high amount of atoms. Mole concept a mole is defined as the amount of a substance that contains exactly the avogadro number of ‘elementary entities’ of the given substance. the avogadro number is represented by na. the mole concept is a convenient method of expressing the amount of a substance. to learn more about the mole concept with formulae and examples with videos and faqs, the number of electrons in a.
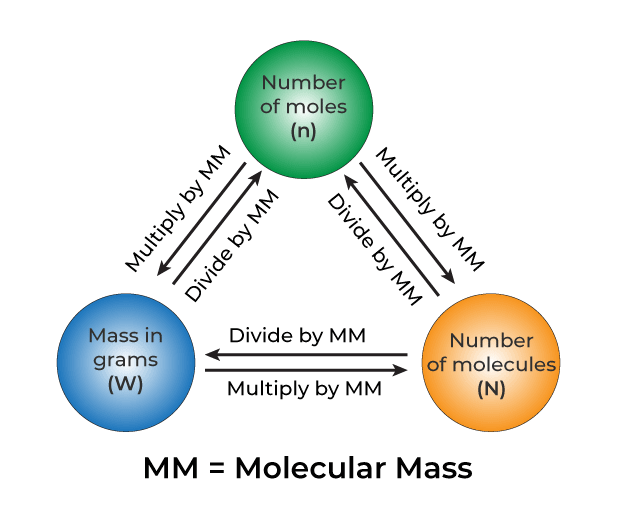
Mole Concept Definition Formula Examples And Faqs The mole, which remains crucial to modern chemical science, was introduced as a unit of the number of substances because of this experimental approach. the mole concept basics of a substance is equal to 12 grams of pure 12 c with the same number of discrete substances (atoms, molecules, ions, etc.) as the number of atoms in that sample. A mole is defined as the amount of substance containing the same number of discrete entities (atoms, molecules, ions, etc.) as the number of atoms in a sample of pure 12c weighing exactly 12 g. one latin connotation for the word “mole” is “large mass” or “bulk,” which is consistent with its use as the name for this unit. The mole is how we relate the unbelievably small atoms and molecules that make something up to the measurable properties such as mass which we may observe in a laboratory setting. a proper understanding of the mole concept is essential in order to do any calculations on experimental data. the mole concept is introduced in this chapter, and will. Calculation of mole concept. we can understand the concept of mole with the help of the below examples. example 1: find the amount of substance in sodium chloride (nacl) when the mass is 92 g. solution: step 1: molecular weight of nacl. na = 23 g mol. cl = 35.5 g mol. molecular weight = 23 35.5 = 58.5 g mol. step 2: by using the formula.

Comments are closed.