Metallic Bond Properties Examples Explanation Britannica 50 Off

Metallic Bond Properties Examples Explanation Britannica Metallic bond, force that holds atoms together in a metallic substance. such a solid consists of closely packed atoms. in most cases, the outermost electron shell of each of the metal atoms overlaps with a large number of neighbouring atoms. as a consequence, the valence electrons continually move from one atom to another and are not associated. Metallic bonds impart several important properties to metals that make them commercially desirable. some of these properties are briefly described in this subsection. 1. electrical conductivity. electrical conductivity is a measure of the ability of a substance to allow a charge to move through it.
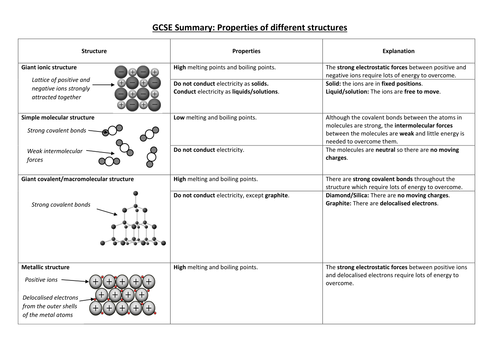
Metallic Bond Properties Examples Explanation Britannica 50ођ Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. these free electrons are called delocalized because they are not confined (localized) to one atom. in contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic bond. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. in contrast, covalent and ionic bonds form between two discrete atoms. metallic bonding is the main type of chemical bond that forms between metal atoms. metallic bonds are seen in pure metals and. The metallic bond is commonly observed in metals. here are some examples [2 4]: 1. sodium (na) sodium has a lone electron in its outermost orbital, i.e., the 3s orbital. when sodium atoms arrange together, the outermost electron of one atom shares space with the corresponding electron on a neighboring atom. The metallic bonding (electron sea model) can explain the physical properties of metals. such as, 1. ductile and malleable. ductility is property of metals for what one can apply stress onto a metal to make it longer or wider without breaking. here (a) is brittle, (b) is partially ductile and (c) is completely ductile in nature.

Metallic Bond Properties Examples Explanation Britannica 50ођ The metallic bond is commonly observed in metals. here are some examples [2 4]: 1. sodium (na) sodium has a lone electron in its outermost orbital, i.e., the 3s orbital. when sodium atoms arrange together, the outermost electron of one atom shares space with the corresponding electron on a neighboring atom. The metallic bonding (electron sea model) can explain the physical properties of metals. such as, 1. ductile and malleable. ductility is property of metals for what one can apply stress onto a metal to make it longer or wider without breaking. here (a) is brittle, (b) is partially ductile and (c) is completely ductile in nature. T. e. metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. it may be described as the sharing of free electrons among a structure of positively charged ions (cations). Metallic bonds occur among metal atoms. whereas ionic bonds join metals to non metals, metallic bonding joins a bulk of metal atoms. a sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. the "sea of electrons" is free to flow about the crystal of positive metal ions.
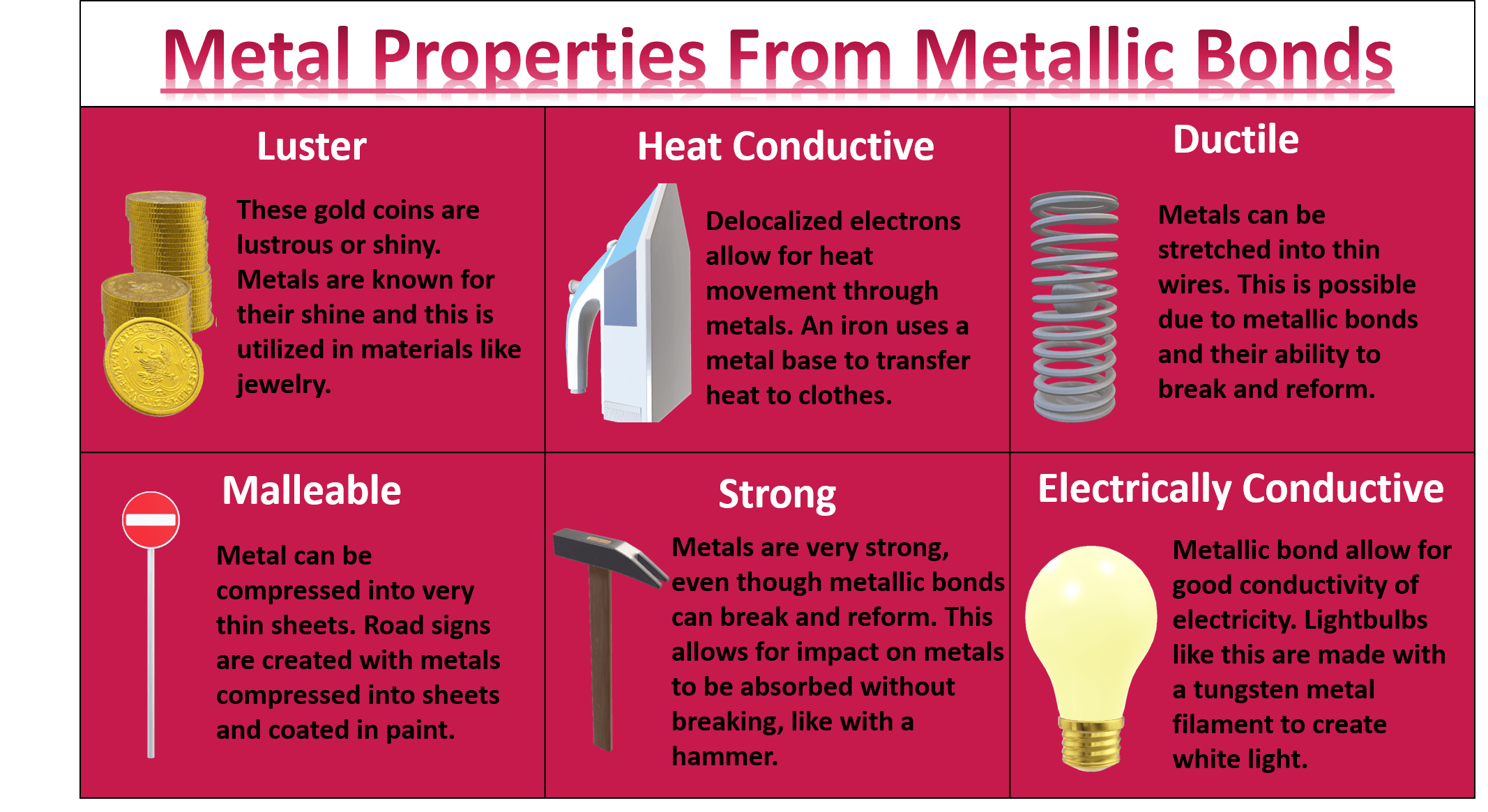
Metallic Bond Examples List T. e. metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. it may be described as the sharing of free electrons among a structure of positively charged ions (cations). Metallic bonds occur among metal atoms. whereas ionic bonds join metals to non metals, metallic bonding joins a bulk of metal atoms. a sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. the "sea of electrons" is free to flow about the crystal of positive metal ions.

Comments are closed.