Metallic Bond Properties Examples Explanation Britannica

Metallic Bond Properties Examples Explanation Britannica Metallic bond, force that holds atoms together in a metallic substance. the outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal. this accounts for many characteristic properties of metals: conductivity, malleability, and ductility. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. the binding arises from the electrostatic attraction of their nuclei for the same electrons. a covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms.
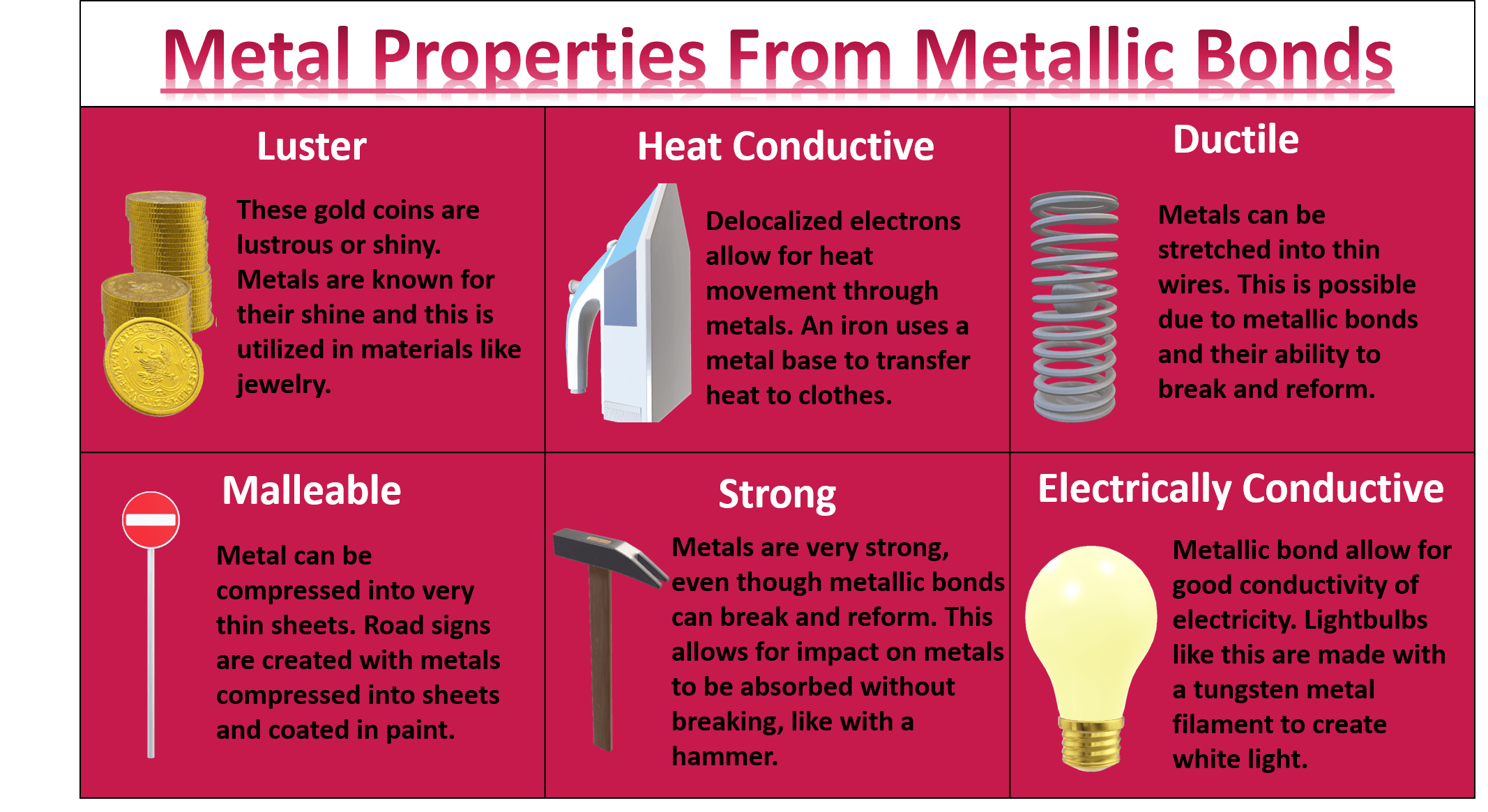
Metallic Bond Examples List Metallic bonds impart several important properties to metals that make them commercially desirable. some of these properties are briefly described in this subsection. 1. electrical conductivity. electrical conductivity is a measure of the ability of a substance to allow a charge to move through it. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. these free electrons are called delocalized because they are not confined (localized) to one atom. in contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic bond. Metallic bonding is the main type of chemical bond that forms between metal atoms. metallic bonds are seen in pure metals and alloys and some metalloids. for example, graphene (an allotrope of carbon) exhibits two dimensional metallic bonding. metals, even pure ones, can form other types of chemical bonds between their atoms. It is a widely distributed mineral known for the beautiful development and great variety of its crystals. calcium does not occur naturally in the free state, but compounds of the element are widely distributed. one calcium compound, lime (calcium oxide, cao) was extensively used by the ancients. the silvery, rather soft, lightweight metal.

Metallic Bonds Properties Examples Explanation Of Metallic Bo Metallic bonding is the main type of chemical bond that forms between metal atoms. metallic bonds are seen in pure metals and alloys and some metalloids. for example, graphene (an allotrope of carbon) exhibits two dimensional metallic bonding. metals, even pure ones, can form other types of chemical bonds between their atoms. It is a widely distributed mineral known for the beautiful development and great variety of its crystals. calcium does not occur naturally in the free state, but compounds of the element are widely distributed. one calcium compound, lime (calcium oxide, cao) was extensively used by the ancients. the silvery, rather soft, lightweight metal. The metallic bond is commonly observed in metals. here are some examples [2 4]: 1. sodium (na) sodium has a lone electron in its outermost orbital, i.e., the 3s orbital. when sodium atoms arrange together, the outermost electron of one atom shares space with the corresponding electron on a neighboring atom. The electrons are said to be delocalized. the metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons. figure 5.7.1: delocaized electrons are free to move in the metallic lattice. this is sometimes described as "an array of positive ions in a sea of electrons".

Comments are closed.