Mechanism Challenge 1 Epoxide Formation вђ Organic Chemistry Tutor
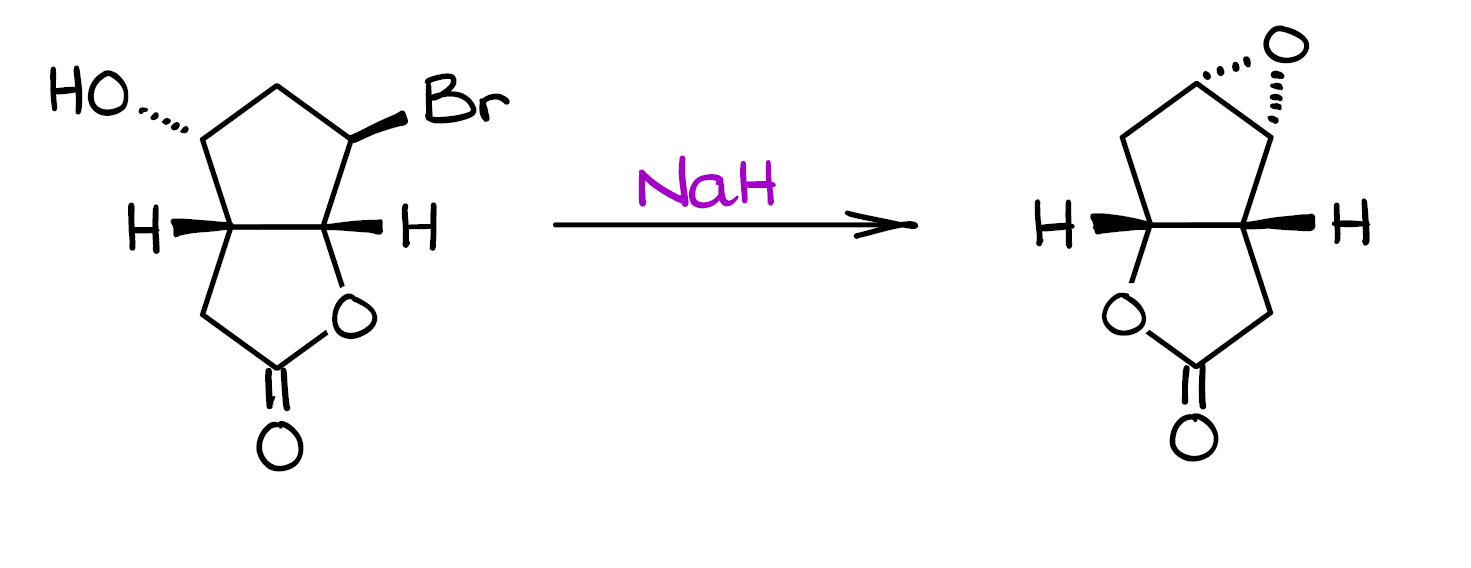
Mechanism Challenge 1 Epoxide Formation вђ Organic Chemistryо Formation of the epoxide. and finally, we can have the sn2 reaction between the new nucleophile that we just made and c7 with the leaving group (br). this gives us our final product—epoxide. and this concludes this entire mechanism. so, as you can see, nothing magically jumps around or changes locations without any obvious reason. Organic chemistry ethers and epoxides. lesson content. 0% complete 0 4 steps. williamson ether synthesis. cleavage of ethers with acids. synthesis of epoxides. epoxide opening. previous topic. back to course.
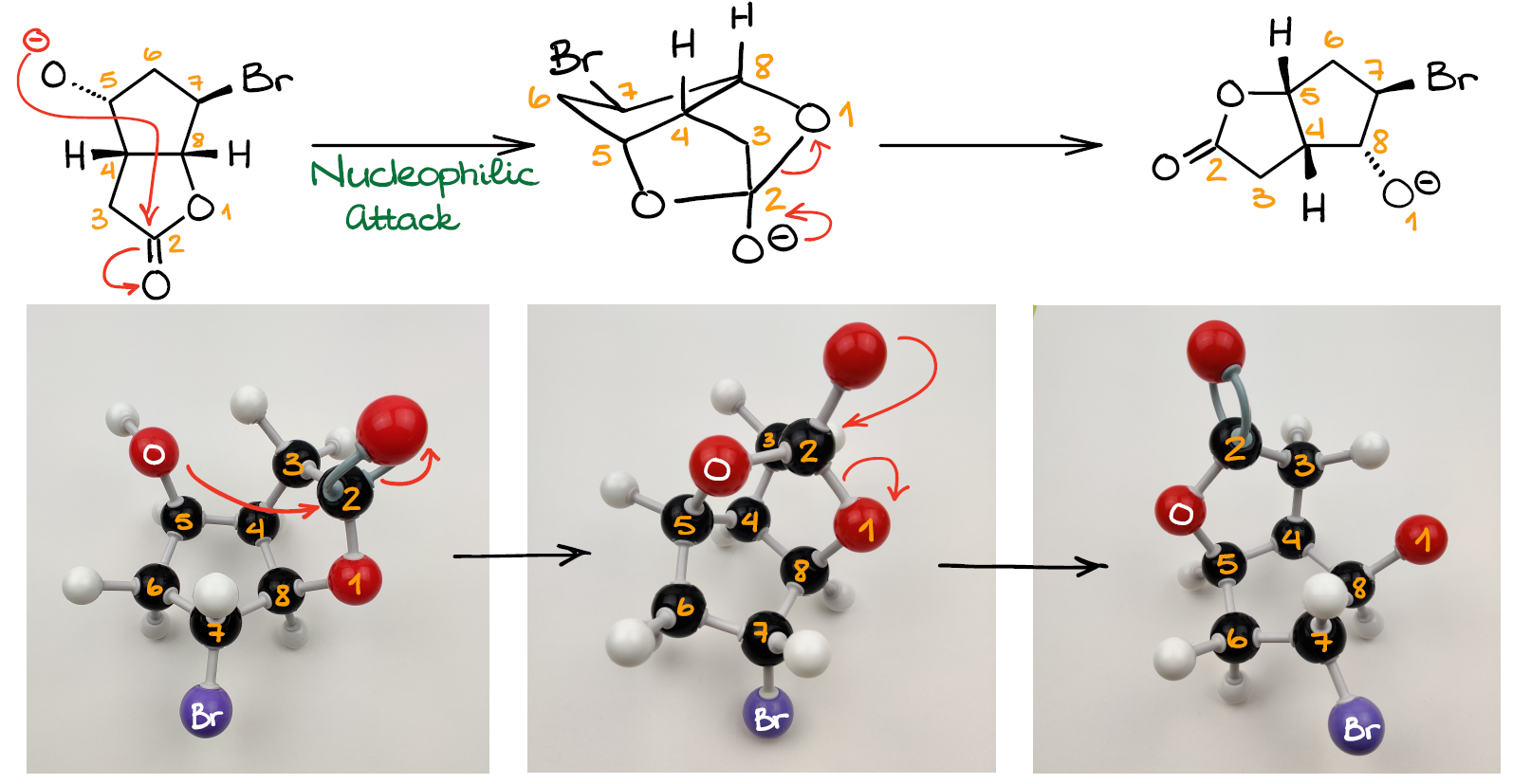
Mechanism Challenge 1 Epoxide Formation вђ Organic Chemistryо Synthesis of epoxides. in this tutorial, we’re delving into the world of epoxide synthesis, an important topic in organic chemistry. epoxides, with their unique three membered ring structure, play a pivotal role in various chemical reactions and are essential in both academic studies and industrial applications. Adding two hydroxyls to opposite faces of an alkene double bond via an epoxide intermediate. created by jay.watch the next lesson: khanacademy.or. Mayr (lmu, germany) is well known for his extremely rigorous work in physical organic chemistry, where he has developed scales for quantifying electrophilicity and nucleophilicity. epoxide ring opening with base can be performed with hydroxide ion, alkoxides, grignard reagents and several other nucleophiles. it proceeds through sn2. 15.7: synthesis of epoxides. page id. epoxides (also known as oxiranes) are three membered ring structures in which one of the vertices is an oxygen and the other two are carbons. the most important and simplest epoxide is ethylene oxide which is prepared on an industrial scale by catalytic oxidation of ethylene by air.
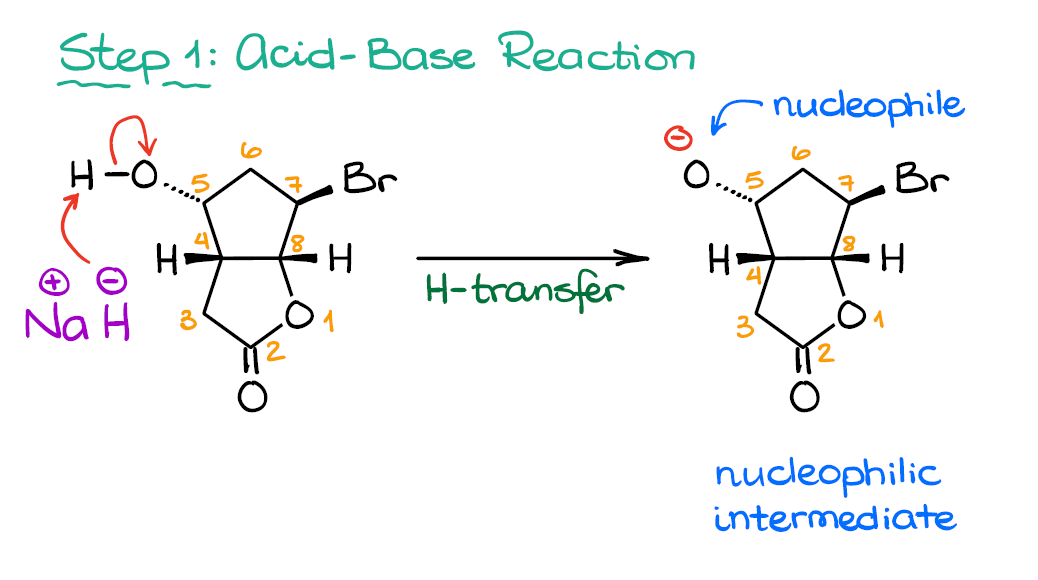
Mechanism Challenge 1 Epoxide Formation вђ Organic Chemistryо Mayr (lmu, germany) is well known for his extremely rigorous work in physical organic chemistry, where he has developed scales for quantifying electrophilicity and nucleophilicity. epoxide ring opening with base can be performed with hydroxide ion, alkoxides, grignard reagents and several other nucleophiles. it proceeds through sn2. 15.7: synthesis of epoxides. page id. epoxides (also known as oxiranes) are three membered ring structures in which one of the vertices is an oxygen and the other two are carbons. the most important and simplest epoxide is ethylene oxide which is prepared on an industrial scale by catalytic oxidation of ethylene by air. Epoxide structure. epoxides (also known as oxiranes) are three membered ring structures in which one of the vertices is an oxygen and the other two are carbons. the carbons in an epoxide group are very reactive electrophiles, due in large part to the fact that substantial ring strain is relieved when the ring opens upon nucleophilic attack. Thus, 1,2 epoxypropane reacts with hcl to give primarily 1 chloro 2 propanol, but 2 methyl 1,2 epoxypropane gives 2 chloro 2 methyl 1 propanol as the major product. the mechanisms of these acid catalyzed epoxide openings are more complex than they initially appear.

Comments are closed.