Manufacturing Process Transfers In The Pharmaceutical Industry A Best

Schematic Block Diagram Of A Pharmaceutical Manufacturing Process Manufacturing process transfers in the pharmaceutical industry: a best practice approach defining technology transfer. according to annex 7 of the world health organization (who) guidelines on the transfer of technology in pharmaceutical manufacturing, technology transfer is defined as “a logical procedure that controls the transfer of any process together with its documentation and. Step 2: process facility fit assessment. the second step is the process and facility fit assessment. ultimately the process must be transferred and manufactured at a commercial site. effective teamwork and knowledge sharing between development and manufacturing considerably streamlines the transfer.
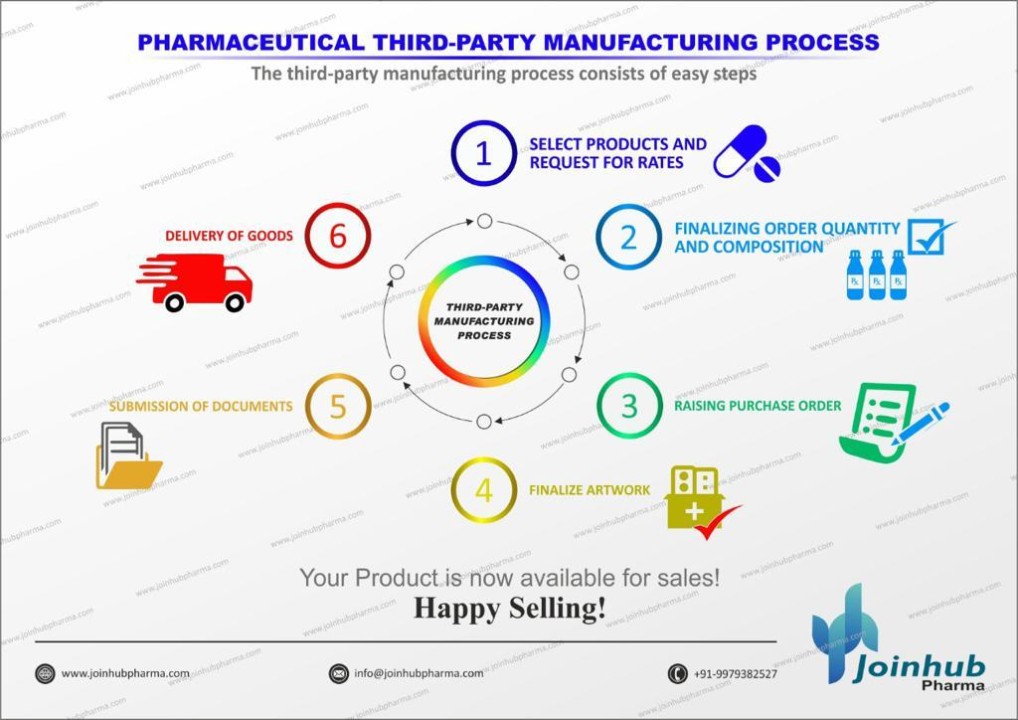
A Complete Guide Of Pharma Manufacturing Process In the pharmaceutical industry a technology transfer more commonly known as a tech transfer – is a series of knowledge transfers on a drug product and its established manufacturing processes from development to commercial production. the ich q10 guidance explains the purpose of technology transfer as follows:. Pharmaceutical quality systems “a common thread to tech transfer”. ich q10 “the change management system should provide management and documentation of adjustments made to the process during technology transfer activities.”. “aspects of management review should be performed to ensure the developed product and process can be. A pharmaceutical technology transfer or more commonly known as a tech transfer, is a series of knowledge transfers on a drug product and its established manufacturing processes from development to commercial production. it can also mean knowledge transfer about existing products from one manufacturing site to another by a facility change, a company merger, an acquisition, or. Technology transfer is the systematic movement of scientific methods and manufacturing processes from development to production or between sites, ensuring product consistency, quality, and regulatory compliance. this process is critical for producing pharmaceuticals safely and cost effectively across various locations and scales, maintaining.

The Benefits Of Automation In Pharmaceutical Manufacturing A pharmaceutical technology transfer or more commonly known as a tech transfer, is a series of knowledge transfers on a drug product and its established manufacturing processes from development to commercial production. it can also mean knowledge transfer about existing products from one manufacturing site to another by a facility change, a company merger, an acquisition, or. Technology transfer is the systematic movement of scientific methods and manufacturing processes from development to production or between sites, ensuring product consistency, quality, and regulatory compliance. this process is critical for producing pharmaceuticals safely and cost effectively across various locations and scales, maintaining. Possible update of the who guidelines on transfer of technology in pharmaceutical manufacturing (1). this original document was published in 2011, since when numerous regulatory changes have been made. transfer of technology is considered an integral part of the product life cycle management and is subject to. Pharmaceutical technology transfer is a dynamic process that requires cross functional collaboration, rigorous documentation, and a commitment to maintaining product quality and patient safety.
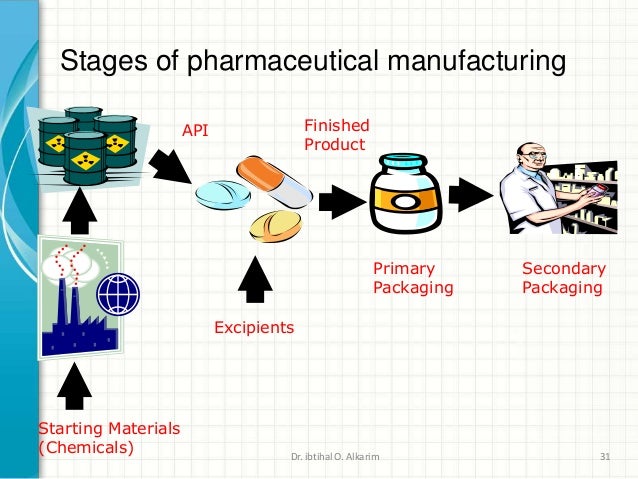
Pharmaceutical Industry And Unit Process Possible update of the who guidelines on transfer of technology in pharmaceutical manufacturing (1). this original document was published in 2011, since when numerous regulatory changes have been made. transfer of technology is considered an integral part of the product life cycle management and is subject to. Pharmaceutical technology transfer is a dynamic process that requires cross functional collaboration, rigorous documentation, and a commitment to maintaining product quality and patient safety.

Comments are closed.