Lewis Structure Sulfur Dioxide Resonance Molecule Sulfur Trioxide
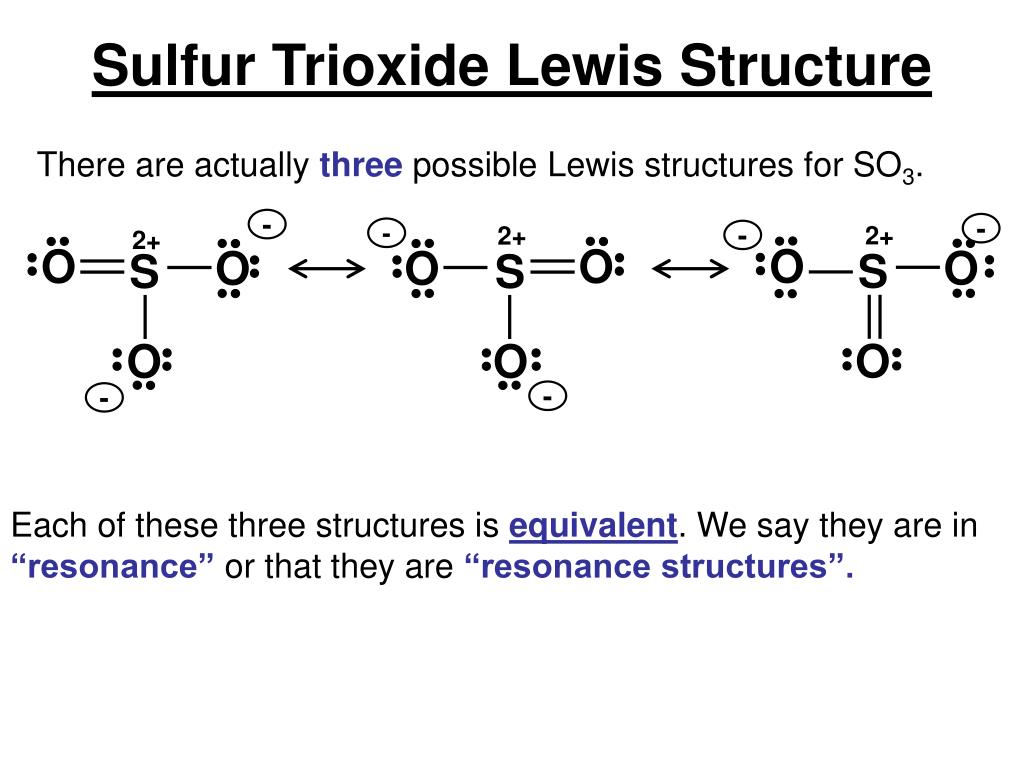
Ppt Chapter 8 Powerpoint Presentation Free Download Id 6001268 Step by step guide to drawing the lewis structure of so3. 1. identify the molecule and count valence electrons. recognize that you are drawing the lewis structure for so3, which consists of one sulfur (s) atom and three oxygen (o) atoms. sulfur has 6 valence electrons, and each oxygen has 6 valence electrons. Sulfur trioxide molecule contains one sulfur atom and three oxygen atoms. we will construct the lewis structure of so 3 molecule by following vsepr theory rules and considering stability of intermediate structures. finally, after obtaining the lewis structure of so 3, we can determine the hybridization of atoms.
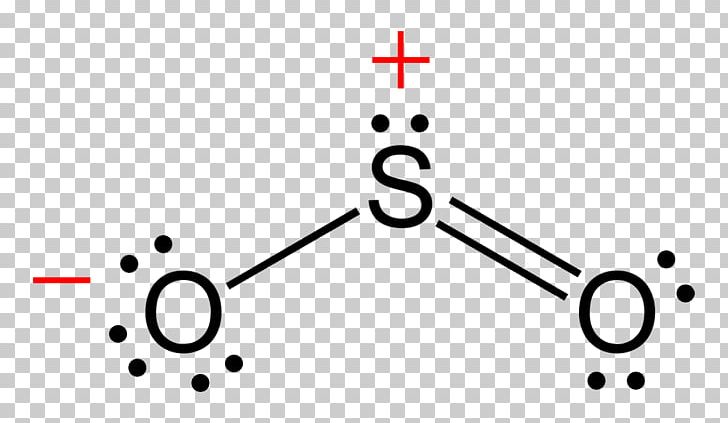
Lewis Structure Sulfur Dioxide Resonance Sulfur Trioxide P There are three resonance structures so3 (sulfur trioxide). we start with a valid lewis structure and then follow these general rules. note that so3 is a bi. How to draw the lewis structure of so3 (sulfur trioxide) with explanationsulfur is an exception to the octet rule it can handle up to 12 electrons!check. As a simplistic explanation, the above sources state that the lewis structure of sox3 s o x 3 contains a 2 charge on the central sulfur and negative charges on two of the three bonded oxygen atoms. in that case, sox3 s o x 3 contains one double bond and two single bonds, which is why people tend to list the overall bond order as 1.33. This chemistry video explains how to draw the lewis structure of so3 sulfur trioxide. it discusses the molecular geometry, bond angle, hybridization, and.
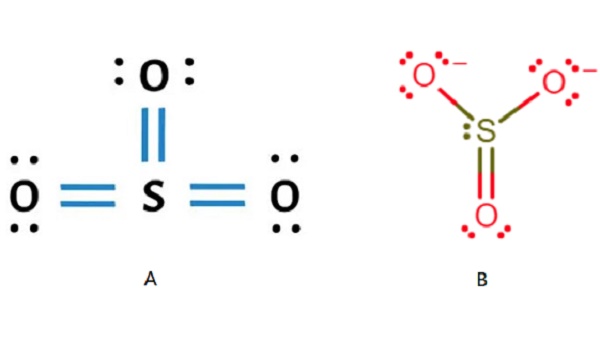
The Lewis Structure Of Sulfur Trioxide Chemicalbook As a simplistic explanation, the above sources state that the lewis structure of sox3 s o x 3 contains a 2 charge on the central sulfur and negative charges on two of the three bonded oxygen atoms. in that case, sox3 s o x 3 contains one double bond and two single bonds, which is why people tend to list the overall bond order as 1.33. This chemistry video explains how to draw the lewis structure of so3 sulfur trioxide. it discusses the molecular geometry, bond angle, hybridization, and. So the resonance structure on the left, and the resonance structure on the right, and some people disagreed with me, and said that's not the dot structure for sulfur dioxide. the dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double. The hybridisation of sulfur trioxide (so3) the hybridization of so3 is sp2. it is determined with the help of formula: number of hybrid orbitals = number of sigma bonds number of lone pairs. in a single shared double covalent bond, there exists one sigma (σ) bond and one pi (π) bond. so, the total number of sigma bonds in a single so3.

Comments are closed.