Lewis Structure Of Sulfur Dioxide So2

Lewis Structure Of Sulphur Dioxide So2 Youtube How to draw the lewis structure of so2 with explanationcheck me out: chemistnate. So the resonance structure on the left, and the resonance structure on the right, and some people disagreed with me, and said that's not the dot structure for sulfur dioxide. the dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double.
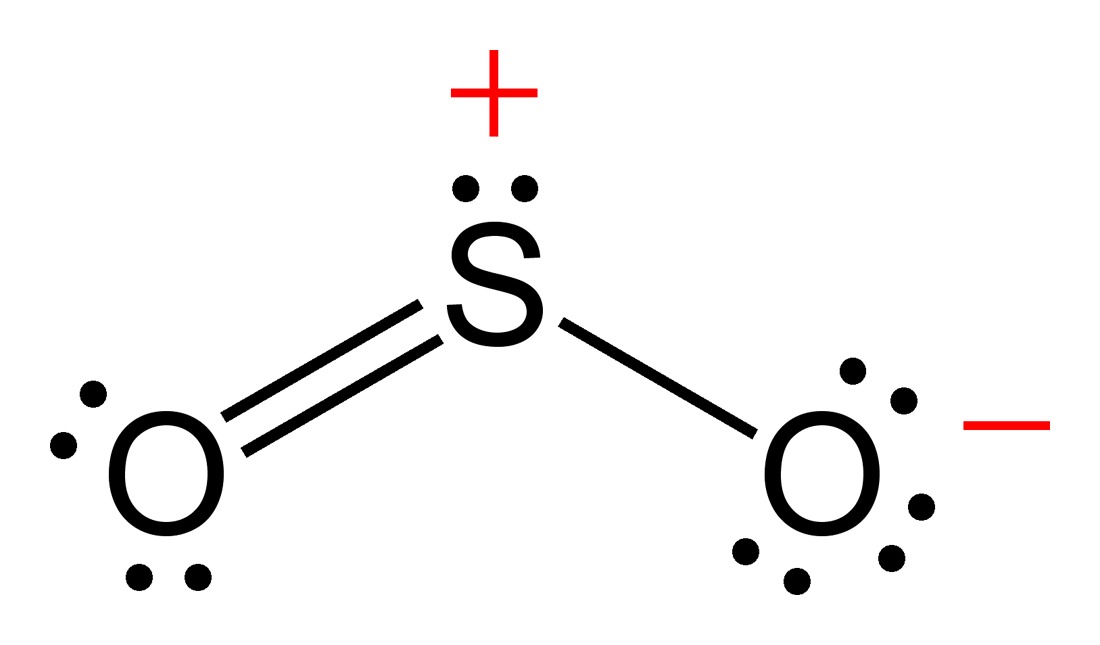
So2 Sulfur Dioxide Molecular Geometry Lewis Structure Geometry Of This chemistry video tutorial explains how to draw the lewis structure of so2 also known as sulfur dioxide. it discusses the molecular geometry, bond angle,. This is a theoretical structure obtained using formal charges this is the structure that we will take to be sulfur dioxide’s final lewis structure. however, it is worth noting that in an experimental sense (data and tools), we find single and double bonds present in the so 2 structure. the final lewis structure for so 2 is shown below. it. 2. ) lewis structure, hybridization. sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. we will construct the lewis structure of so 2 molecule by following vsepr theory rules and considering stability of intermediate structures. after obtaining the lewis structure of so 2, we can determine the hybridization of atoms. Today in this video, we will determine the lewis dot structure for sulphur dioxide, having a chemical formula of so2. it comprises one sulphur atom and two o.

So2 Lewis Structure How To Draw The Lewis Structure For So2 S 2. ) lewis structure, hybridization. sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. we will construct the lewis structure of so 2 molecule by following vsepr theory rules and considering stability of intermediate structures. after obtaining the lewis structure of so 2, we can determine the hybridization of atoms. Today in this video, we will determine the lewis dot structure for sulphur dioxide, having a chemical formula of so2. it comprises one sulphur atom and two o. Sulfur dioxide is spelled as sulphur dioxide in commonwealth english. this is a pungent smelling, colorless gas. talking about its properties, so2 has a molar mass of 64.066 g mol. the melting point and boiling points are 72℃, and 10℃ respectively. now let’s move on to the fundamental concepts like lewis structure, molecular geometry. Subtract step 1 total from step 2. 24 18=6e . step 4: find number of bonds by diving the number in step 3 by 2 (because each bond is made of 2 e ) 6e 2= 3 bond pairs. step 5: find the number of nonbonding (lone pairs) e . subtract step 3 number from step 1. 18 6= 12e =6 lone pairs. now, use the information from step 4 and 5 to draw the lewis.

Comments are closed.