Lewis Diagrams And Vsepr Models
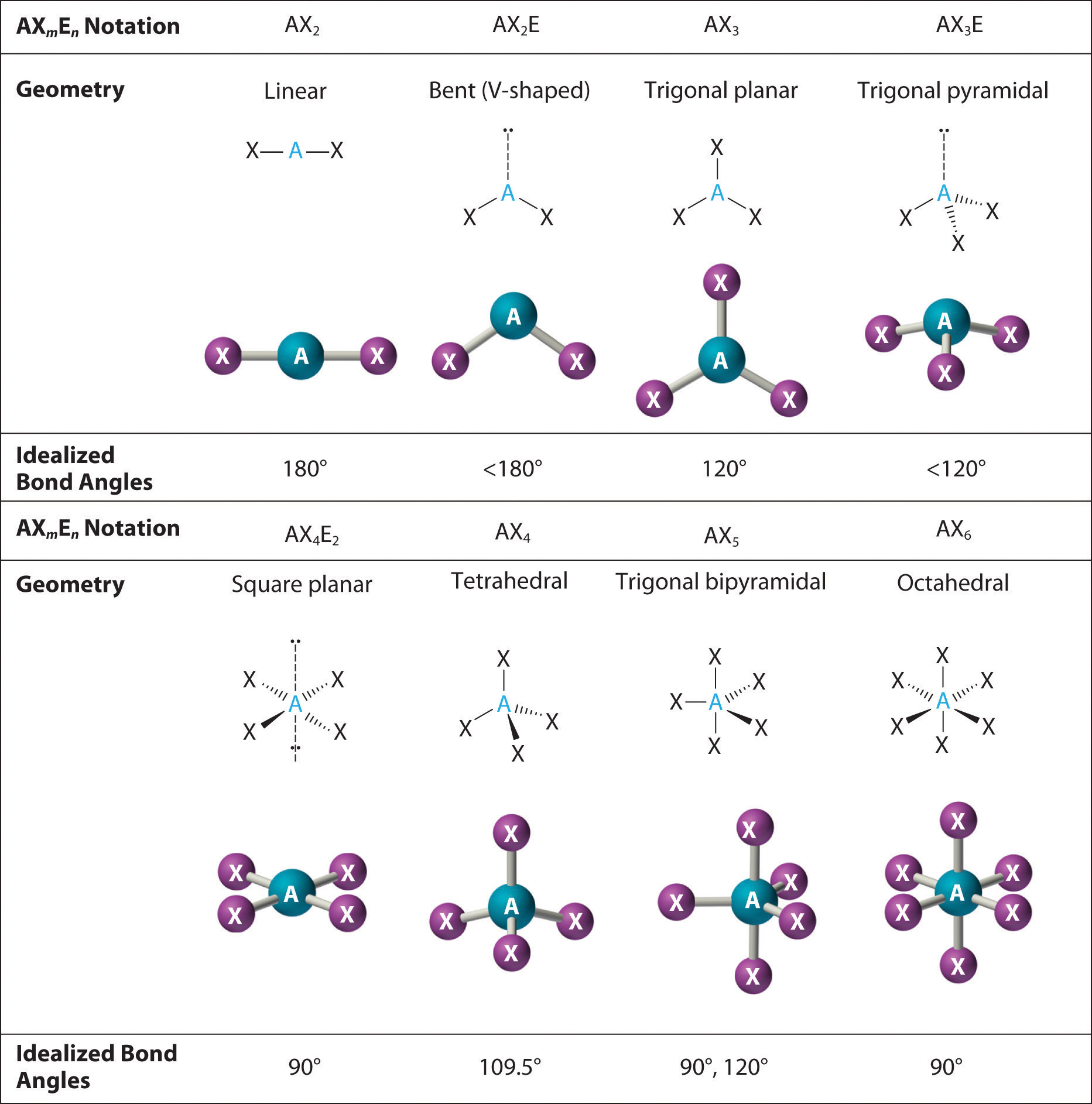
9 2 The Vsepr Model Chemistry Libretexts 2. the carbon atom forms two double bonds. each double bond is a group, so there are two electron groups around the central atom. like beh 2, the arrangement that minimizes repulsions places the groups 180° apart. 3. once again, both groups around the central atom are bonding pairs (bp), so co 2 is designated as ax 2. 022 lewis diagrams and vsepr modelsin this video paul andersen explains how you can use lewis diagrams and vsepr models to make predictions about molecules.
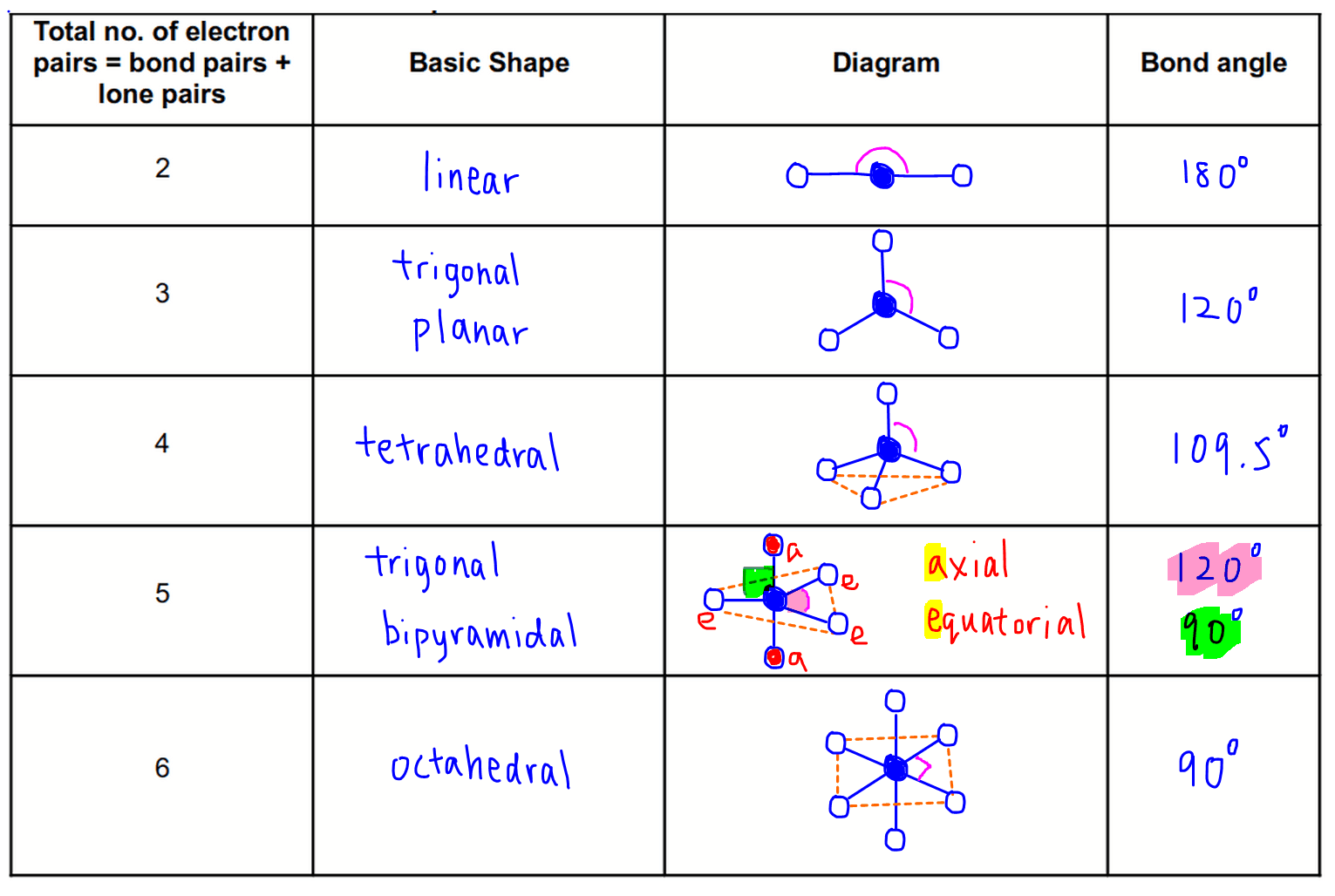
Vsepr Theory And Shapes Of Molecules Valence shell electron pair repulsion theory (vsepr theory) enables us to predict the molecular geometry, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its lewis structure. the vsepr model assumes that electron pairs in the valence shell of a central. Lecture video. valence shell electron pair repulsion or vsepr theory can be used to predict molecular geometry. the theory is based on lewis structures and the simple idea that that the preferred geometry around a central atom is the one that minimizes electron repulsion. The vsepr model. the vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. the vsepr model is not a theory; it does not attempt to explain observations. Each fluorine atom give 1 electron to the phosphorus central atom which creates total of 5 pairs. also, each fluorine atom has 3 electron pairs. with the presence of 5 fluorine atom, there are 15 more electron pairs so there are 20 electron pairs total. 13.13: molecular structure: the vsepr model is shared under a license and was authored.

Vsepr Theory Chart The vsepr model. the vsepr model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. the vsepr model is not a theory; it does not attempt to explain observations. Each fluorine atom give 1 electron to the phosphorus central atom which creates total of 5 pairs. also, each fluorine atom has 3 electron pairs. with the presence of 5 fluorine atom, there are 15 more electron pairs so there are 20 electron pairs total. 13.13: molecular structure: the vsepr model is shared under a license and was authored. The vsepr theory therefore predicts that co 2 will be a linear molecule, just like bef 2, with a bond angle of 180 o. the lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. but these electrons are concentrated in three places: the two c o single bonds and the c=o double bond. The valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced "vesper") and is a model to predict the geometry of molecules. specifically, vsepr models look at the bonding and molecular geometry of organic molecules and polyatomic ions. it is useful for nearly all compounds that have a central atom that is not a metal. lewis structures only tell the number.

Shape Of Molecules Vsepr Theory Affect Shape Of The Molecule The vsepr theory therefore predicts that co 2 will be a linear molecule, just like bef 2, with a bond angle of 180 o. the lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. but these electrons are concentrated in three places: the two c o single bonds and the c=o double bond. The valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced "vesper") and is a model to predict the geometry of molecules. specifically, vsepr models look at the bonding and molecular geometry of organic molecules and polyatomic ions. it is useful for nearly all compounds that have a central atom that is not a metal. lewis structures only tell the number.

Vsepr Theory Explanation Chart And Examples

Comments are closed.