Lecture 4 Browning Reaction
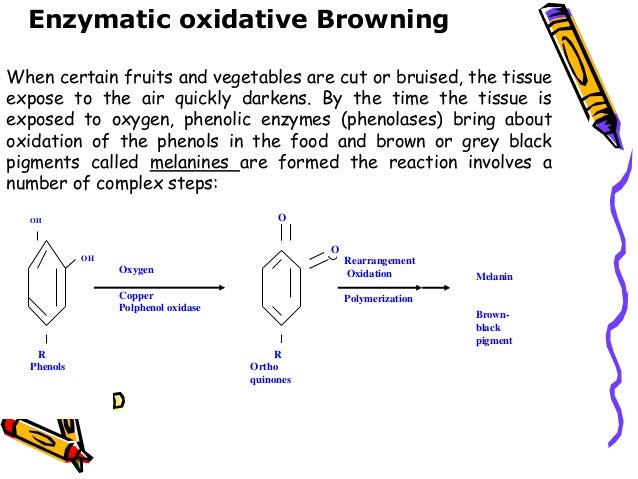
Lecture 4 Browning Reaction Lecture 4 browning reaction. this document summarizes the two main types of browning reactions: non enzymatic and enzymatic. non enzymatic browning includes caramelization of sugars with heat and the maillard reaction between sugars and amino acids. enzymatic browning is caused by polyphenol oxidase enzymes acting on phenolic compounds in. Summary. browning is one of the most important reactions taking place during food processing and storage. both, enzymatic and nonenzymatic browning can affect the quality of food in either positive or negative ways, depending on the type of food. special attention has been paid in this chapter to chemistry of the reaction.

Lecture 4 Browning Reaction Ppt The maillard reaction is a set of chemical reactions between amino acids and reducing sugars that browns food. named after the french chemist louis camille maillard, who first described it in the early 20th century, this non enzymatic browning reaction is responsible for the flavors, aromas, and colors in a wide range of cooked foods, from. Browning itself is subdivided roughly (again because there is an overlap) into three types of reactions. the first, called the maillard reaction,1 occurs between a carbonyl compound, which here is usually a reducing sugar, and an amine, which here is usually an amino acid, a peptide, or a protein. the second is caramelisation, a reaction where the. Ways to increase maillard reaction. baking soda increases alkalinity, increases carmelization, sweetness. makes onions softer, mushier. brush baked goods with milk or eggs to put necessary protein on the surface for browning. eggs, milk add protein to baked goods. soup stocks and demi glace are boiled for long times to create complex maillard. Derivativeanderythronicacidaremetabolicallyinert, oxidativecleavageofamadoricompoundsinvivomay limitproteinglycation;(b)carboxymethyllysinemaybe.

Lecture 4 Browning Reaction Ways to increase maillard reaction. baking soda increases alkalinity, increases carmelization, sweetness. makes onions softer, mushier. brush baked goods with milk or eggs to put necessary protein on the surface for browning. eggs, milk add protein to baked goods. soup stocks and demi glace are boiled for long times to create complex maillard. Derivativeanderythronicacidaremetabolicallyinert, oxidativecleavageofamadoricompoundsinvivomay limitproteinglycation;(b)carboxymethyllysinemaybe. Maillard reaction is a chemical reaction that occurs between amino acids and reducing sugars. the thermal processing of amino acids and sugars produces a mixture of complex compounds. the reaction is responsible for the non enzymatic browning of food, like a cookie, biscuit, bread, and steak. browning occurs during frying, roasting, searing. One of the most important flavor producing reactions in cooking is the maillard reaction. it is sometimes called the “browning reaction” in discussions of cooking, but that description is incomplete at best. cooked meats, seafood, and other protein laden foods that undergo the maillard reaction do turn brown, but there are other reactions that also cause browning. […].

Comments are closed.