Lab 4 Atoms And Elements

Assignment Lab 4 Atomic Structure And Electron Arrangement Report Pdf For this part of the experiment you will make a graph of a periodic property vs. atomic number. the property chosen is atomic radius, given in pm (1 pm = 10–12m). the atomic radius, r, is related to the volume of the atom by the formula of a sphere, v = 4 3 r3. in table 1 below, the atomic radii of the first 54 elements are given. An atom consists of a small, positively charged nucleus surrounded by electrons. the nucleus contains protons and neutrons; its diameter is about 100,000 times smaller than that of the atom. the mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. an amu is defined as exactly 1 12 1 12 of.

Atomic 4concept Lablearner Study with quizlet and memorize flashcards containing terms like (4.1) write the correct chemical symbols for each of the following elements: a. iodine b. iron c. magnesium d. zinc e. nitrogen, (4.1) give the names of the elements with the following symbols: a. p b. al c. mn d. h e. k, (4.2) identify the element described by each of the following groups and periods: 1. 4.4: protons, neutrons, and electrons. atoms are composed of protons, neutrons, and electrons. electrons are extremely small. the mass of an electron is only about 1 2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. electrons have an electric charge of −1, which is equal but opposite. Find selenium in the periodic table shown in figure 4.6.1 4.6. 1 and then classify the element according to its location. solution: the atomic number of selenium is 34, which places it in period 4 and group 16. in figure 4.6.1 4.6. 1, selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so. For example, atoms of the element hydrogen always have one proton in their nuclei, while atoms of the next element, helium, always have two protons in their nuclei. atoms of the element carbon similarly contain six protons, and atoms of iron have 26 protons. in a neutral atom, the number of protons is equal to the number of electrons.

Chapter 4 Atoms And Elements Section 2 Atomic Find selenium in the periodic table shown in figure 4.6.1 4.6. 1 and then classify the element according to its location. solution: the atomic number of selenium is 34, which places it in period 4 and group 16. in figure 4.6.1 4.6. 1, selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so. For example, atoms of the element hydrogen always have one proton in their nuclei, while atoms of the next element, helium, always have two protons in their nuclei. atoms of the element carbon similarly contain six protons, and atoms of iron have 26 protons. in a neutral atom, the number of protons is equal to the number of electrons. Chem 1105 lab handouts; experiment 4 the periodic table atoms and elements experiment 4 the periodic table atoms and elements to print or download this. The elements in a (mixture, solution, compound) are always combined in the same proportion by mass. the compound ammonia contains three atoms of hydrogen for every atom of nitrogen, so the. chemical formula for ammonia is (nh3, n3h3, n3h). an example of a homogenous mixture is (vegetable soup, air, granite rock).
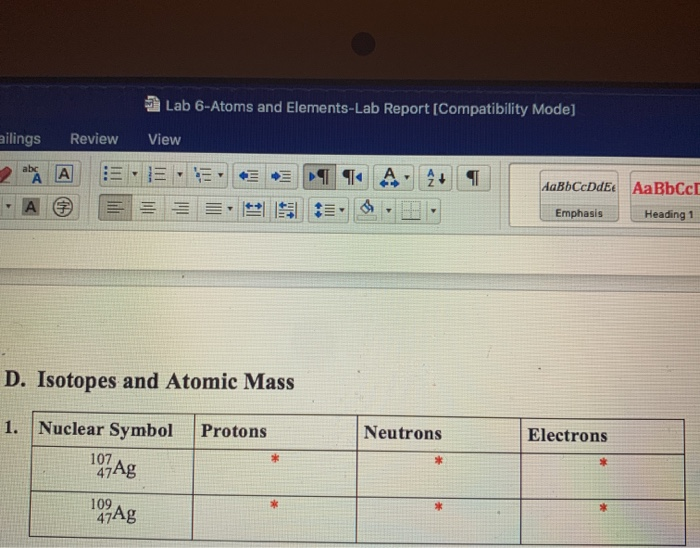
Solved Lab 6 Atoms And Elements Lab Report Compatibility Chegg Chem 1105 lab handouts; experiment 4 the periodic table atoms and elements experiment 4 the periodic table atoms and elements to print or download this. The elements in a (mixture, solution, compound) are always combined in the same proportion by mass. the compound ammonia contains three atoms of hydrogen for every atom of nitrogen, so the. chemical formula for ammonia is (nh3, n3h3, n3h). an example of a homogenous mixture is (vegetable soup, air, granite rock).

Comments are closed.