Kinetics 3 Rate Law

Chemical Kinetics 3 Rate Law And Order Of Reaction Youtube If m = 1 and n = 1, the overall order of the reaction is second order (m n = 1 1 = 2). the rate law: rate = k[h 2o 2] describes a reaction that is first order in hydrogen peroxide and first order overall. the rate law: rate = k[c 4h 6]2. describes a reaction that is second order in c 4 h 6 and second order overall. 3.3.3: reaction order. the reaction order is the relationship between the concentrations of species and the rate of a reaction. the rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of reactants and products.

Kinetics Lesson 3 Rate Law Rate Law Equation Rate equation. in chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an empirical differential mathematical expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and partial orders of reaction) only. Rates of chemical reactions are usually defined by comparing the change in reactant or product concentration over time. consider the reaction n2 3 h2 2 nh3. we could measure the rate at which n2 and h2 are consumed and the rate at which nh3 is produced. due to the stoichiometry of the reaction, the rate of n2 use will be 1 3 the rate of h2. The reaction orders m and n in equation 2.1.2 are not the same as the stoichiometric coefficients and must be determined from experiment. equation 2.1.2 is known as the rate law and the overall reaction order is determined by the sum of the orders n and m for each reactant. we will now consider a few cases. In thinking about chemical reactions, rate matters. this lecture provides an introduction to kinetics and shows one of the coolest reactions known: the oscillating clock reaction. watch as colors change quickly as different steps in the reaction become spontaneous. if playback doesn't begin shortly, try restarting your device.
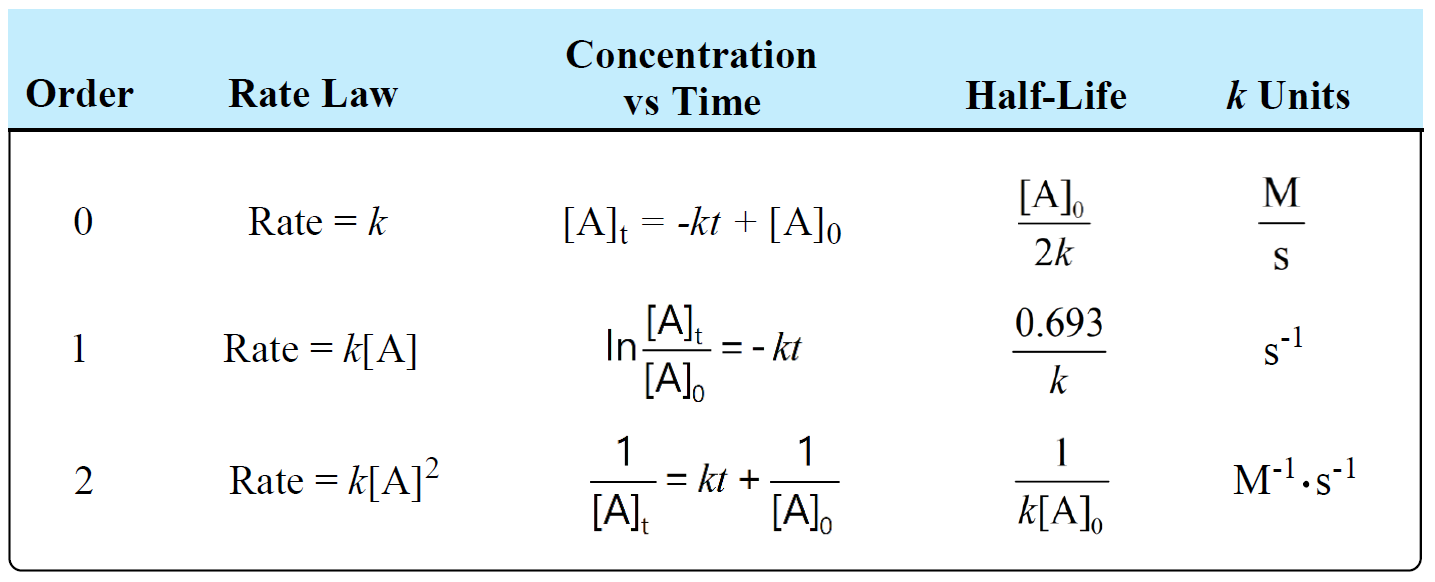
Units Of Rate Constant K Chemistry Steps The reaction orders m and n in equation 2.1.2 are not the same as the stoichiometric coefficients and must be determined from experiment. equation 2.1.2 is known as the rate law and the overall reaction order is determined by the sum of the orders n and m for each reactant. we will now consider a few cases. In thinking about chemical reactions, rate matters. this lecture provides an introduction to kinetics and shows one of the coolest reactions known: the oscillating clock reaction. watch as colors change quickly as different steps in the reaction become spontaneous. if playback doesn't begin shortly, try restarting your device. Referring to the generic rate law above, the reaction is m order with respect to a and n order with respect to b. for example, if m = 1 and n = 2, the reaction is first order in a and second order in b. the overall reaction order is simply the sum of orders for each reactant. for the example rate law here, the reaction is third order overall (1. The solution is actually very simple: the reaction rate is defined as the rate of change of the concentration of a reactant or product divided by its stochiometric coefficient. for the above reaction, the rate (usually given the symbol ν) is therefore. 1 ν d[n2] d[h2] 1 d[nh3] = dt = 3 dt = 2 dt.

Chem 1b Lec 7 Kinetics Part 3 Rate Law Practice Youtube Referring to the generic rate law above, the reaction is m order with respect to a and n order with respect to b. for example, if m = 1 and n = 2, the reaction is first order in a and second order in b. the overall reaction order is simply the sum of orders for each reactant. for the example rate law here, the reaction is third order overall (1. The solution is actually very simple: the reaction rate is defined as the rate of change of the concentration of a reactant or product divided by its stochiometric coefficient. for the above reaction, the rate (usually given the symbol ν) is therefore. 1 ν d[n2] d[h2] 1 d[nh3] = dt = 3 dt = 2 dt.

Comments are closed.