Isentropic Process

Isentropic Process Work Done Efficiency Explanation Eigenplus An isentropic process is a thermodynamic process that is both adiabatic and reversible, with constant entropy. learn about the definition, examples, applications, and derivation of isentropic processes in various systems and cycles. An isentropic process is a thermodynamic process in which the entropy of a fluid or gas remains constant. learn how to calculate the enthalpy change, the speed of sound, and the isentropic efficiency of isentropic processes in various cycles and devices.
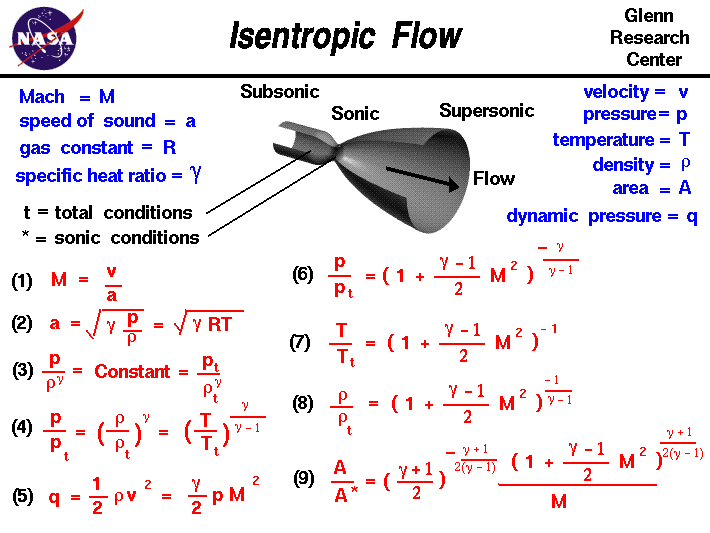
Isentropic Flow Equations Learn what an isentropic process is, how to calculate it, and why it matters in engineering thermodynamics. an isentropic process is an idealized, adiabatic, and reversible process with constant entropy and specific volume. Learn what isentropic process is, how it differs from adiabatic and isothermal processes, and how it applies to thermodynamic cycles and turbines. find out the isentropic relations, flow, efficiency and equations with examples and diagrams. An isentropic process is a reversible adiabatic process in which the entropy of the fluid or gas remains constant. learn how to use enthalpy, ideal gas law and p v diagram to analyze isentropic processes in thermodynamic cycles and devices. Learn what isentropic process means in thermodynamics, how it differs from adiabatic and irreversible processes, and how it applies to ideal gases. explore the concept of entropy, reversibility, and energy transfer with examples and diagrams.
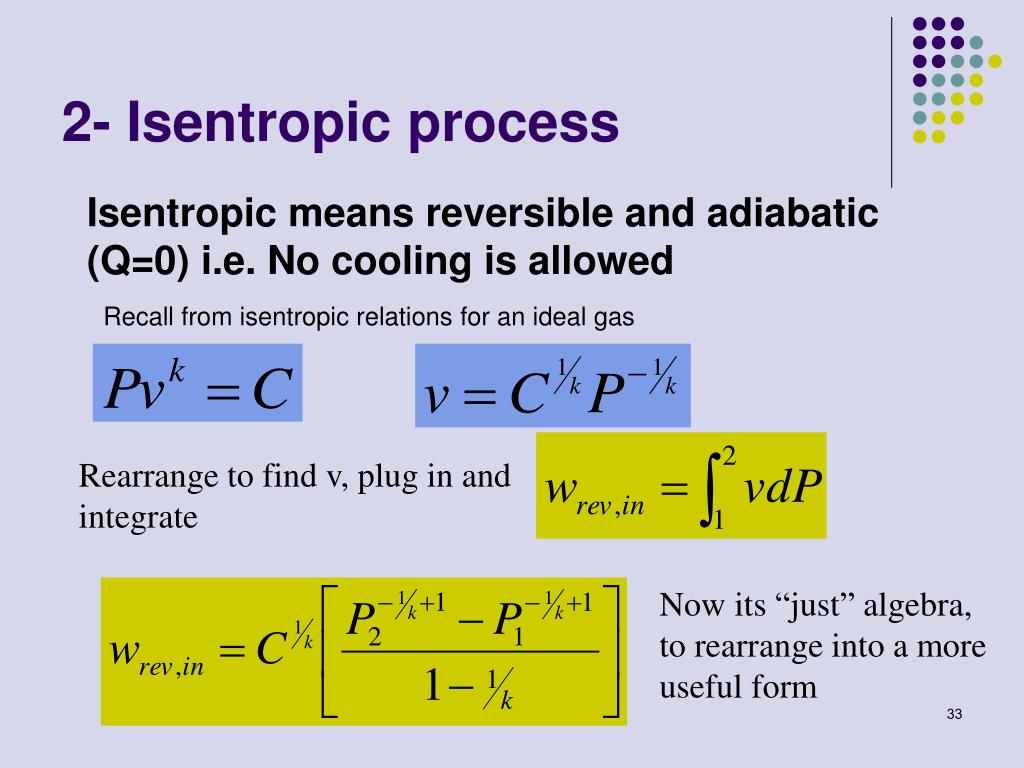
Ppt Entropy Balance For Open Systems Powerpoint Presentation Free An isentropic process is a reversible adiabatic process in which the entropy of the fluid or gas remains constant. learn how to use enthalpy, ideal gas law and p v diagram to analyze isentropic processes in thermodynamic cycles and devices. Learn what isentropic process means in thermodynamics, how it differs from adiabatic and irreversible processes, and how it applies to ideal gases. explore the concept of entropy, reversibility, and energy transfer with examples and diagrams. An isentropic process is a reversible process of an adiabatic system, where no heat is transferred across the system boundary. learn how to achieve an approximately isentropic process, how to illustrate it in the volume pressure diagram, and how to derive the relationships between volume, pressure and temperature. Learn how to calculate the pressure, density, temperature, and speed of an isentropic flow of a gas. use the javascript program to solve the equations and explore the effects of mach number, angle, and area ratio on the flow conditions.

Processing Meaning An isentropic process is a reversible process of an adiabatic system, where no heat is transferred across the system boundary. learn how to achieve an approximately isentropic process, how to illustrate it in the volume pressure diagram, and how to derive the relationships between volume, pressure and temperature. Learn how to calculate the pressure, density, temperature, and speed of an isentropic flow of a gas. use the javascript program to solve the equations and explore the effects of mach number, angle, and area ratio on the flow conditions.

Comments are closed.