Introduction To Phases Of Clinical Trials
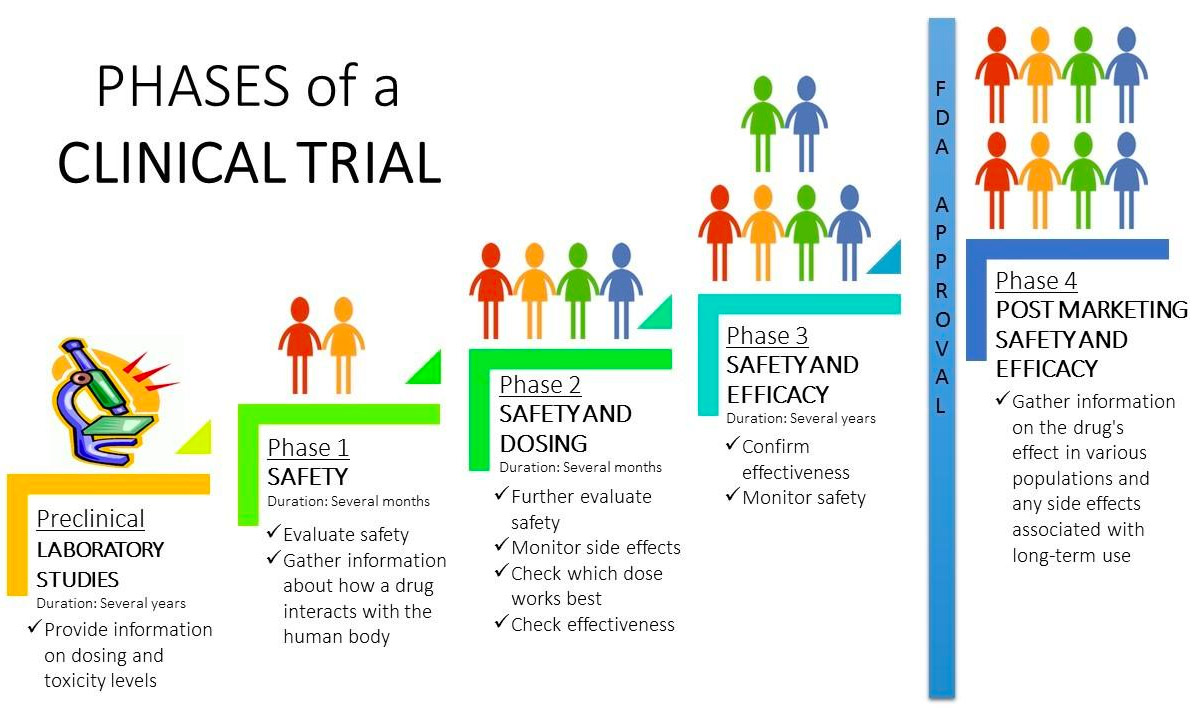
Cancer Clinical Trials Cancer Care St Luke S Cancer Center Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current. Clinical trial includes various phases that include phase 0 (micro dosing studies), phase 1, phase 2, phase 3, and phase 4 . phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post approval or the post marketing.
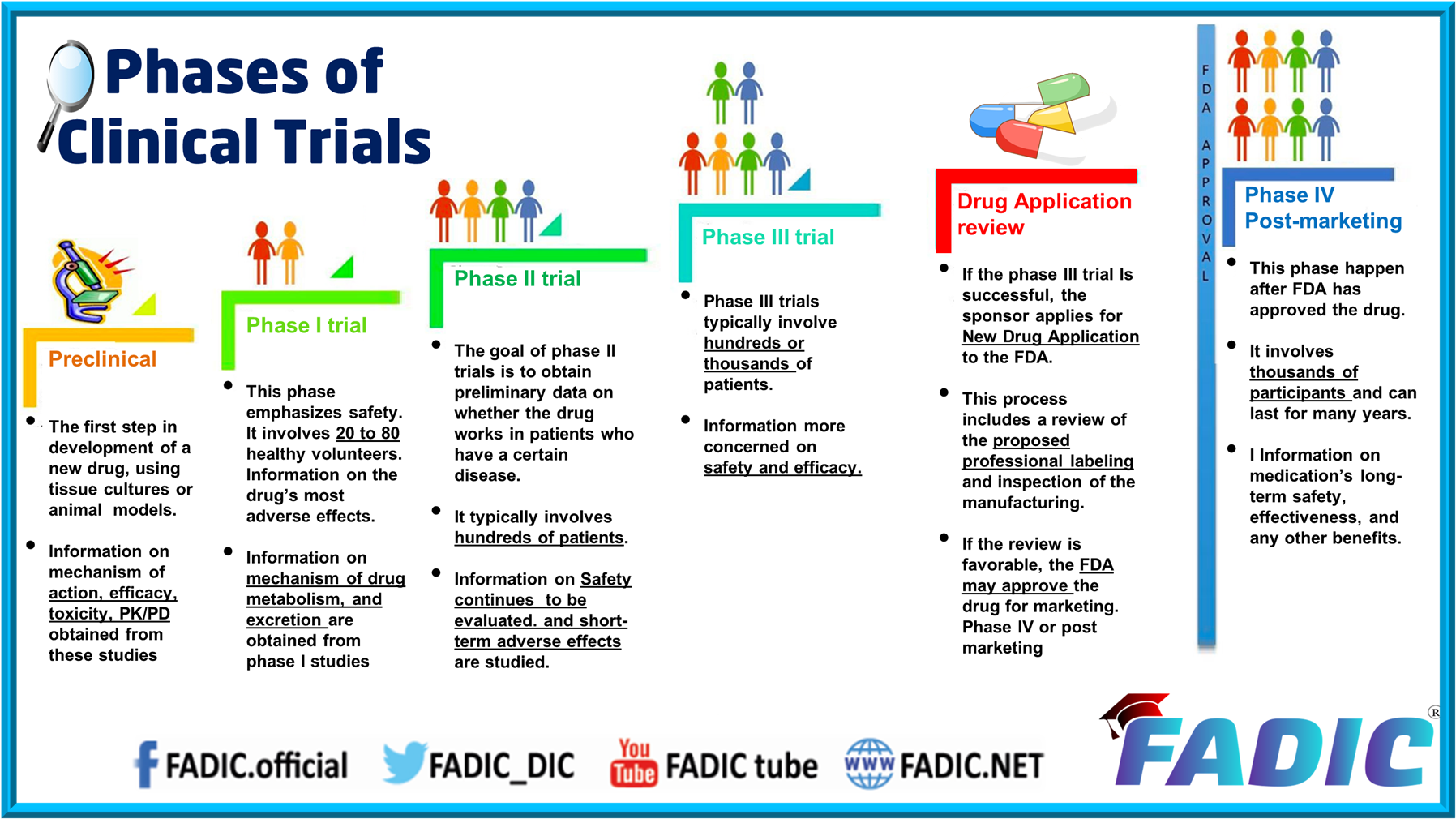
Phases Of Clinical Trials 30 Minutes E Course The drug development process. step 3: clinical research. while preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will. Clinical trials are conducted in a series of steps called “phases.” each phase has a different purpose and helps researchers answer different questions. phase i trials: researchers test a drug or treatment in a small group of people (20–80) for the first time. the purpose is to study the drug or treatment to learn about safety and. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and.
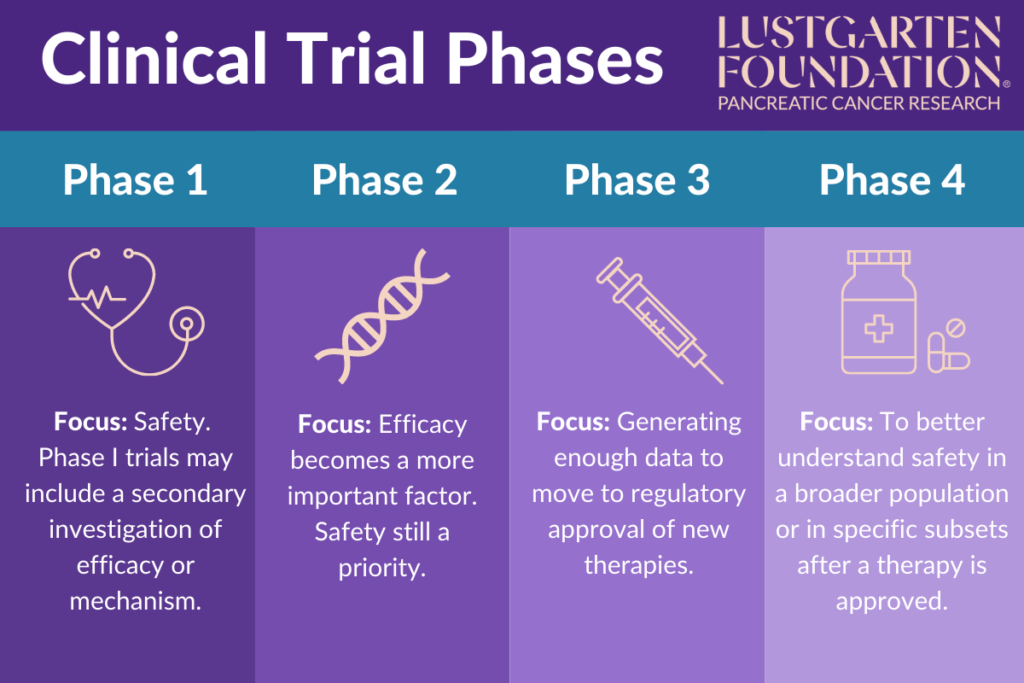
Clinical Trial Phases Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. Fundamental clinical trial design issues are discussed. keywords: p value, confidence intervals, intent to treat, missing data, multiplicity, subgroup analyses, causation. go to: 1. introduction. the objective of clinical trials is to establish the effect of an intervention. treatment effects are efficiently isolated by controlling for bias and. Begin your studies of clinical trials with an introduction to the background and rationale, a review of protocol development and trial registration, and an outline of a trial’s phases and stages. trial design. compare the advantages and disadvantages of different trial types and review three frameworks for clinical trials.

Phases Of Clinical Trials Chart Fundamental clinical trial design issues are discussed. keywords: p value, confidence intervals, intent to treat, missing data, multiplicity, subgroup analyses, causation. go to: 1. introduction. the objective of clinical trials is to establish the effect of an intervention. treatment effects are efficiently isolated by controlling for bias and. Begin your studies of clinical trials with an introduction to the background and rationale, a review of protocol development and trial registration, and an outline of a trial’s phases and stages. trial design. compare the advantages and disadvantages of different trial types and review three frameworks for clinical trials.

Comments are closed.