Ideal Bond Angles вђ Overview Examples Expii

Ideal Bond Angles вђ Overview Examples Expii Text. 1. the ideal bond angles are the angles that would be formed if all of the electron domains surrounding an atom were arranged in a perfectly symmetrical manner. ultimately, these ideal bond angles are usually not quite correct, because lone electron pairs repel other electron pairs more strongly than bonding electron pairs. Water molecules are a great example of bond angle deviation. water is known to have a bent geometry from the two hydrogen bonds to the central oxygen atom and two lone lairs. water is not a linear molecule where the bonding pairs are 180∘ from each other. this is because the repulsion between lone pairs and bonding pairs causes a bent geometry.
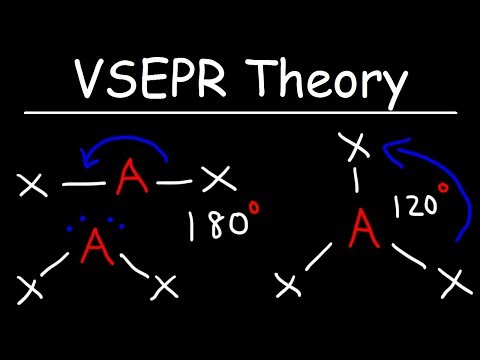
Ideal Bond Angles вђ Overview Examples Expii The three hydrogens angle away, creating a 107 degree angle. the second deviation is the bent geometry. the most famous example is water (h2o). in water, the oxygen atom has two lone pairs that repel the hydrogens. the bond angle in water is 104.5 degrees. we are beginning to have more significant deviations from the ideal bond angles. Because a lone pair is not shared by two nuclei, it occupies more space near the central atom than a bonding pair (figure 10.2.4 10.2. 4). thus bonding pairs and lone pairs repel each other electrostatically in the order bp–bp < lp–bp < lp–lp. in so 2, we have one bp–bp interaction and two lp–bp interactions. 4. Figure 5.2.2 5.2. 2: the bef2 molecule adopts a linear structure in which the two bonds are as far apart as possible, on opposite sides of the be atom. figure 5.2.3 5.2. 3 illustrates this and other electron pair geometries that minimize the repulsions among regions of high electron density (bonds and or lone pairs). Oceanographers study the mixing of water masses by releasing tracer molecules at a site and then detecting their presence at other places. the molecule trifluoromethylsulfur pentafluoride is one such tracer. draw an electron dot structure for cf3sf5, and predict the bond angles around both carbon and sulfur. 383.
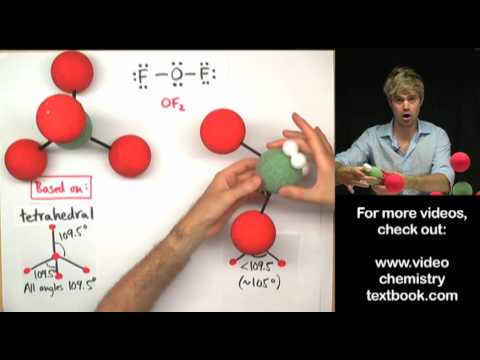
Deviation From Ideal Bond Angles вђ Overview Examples Expii Figure 5.2.2 5.2. 2: the bef2 molecule adopts a linear structure in which the two bonds are as far apart as possible, on opposite sides of the be atom. figure 5.2.3 5.2. 3 illustrates this and other electron pair geometries that minimize the repulsions among regions of high electron density (bonds and or lone pairs). Oceanographers study the mixing of water masses by releasing tracer molecules at a site and then detecting their presence at other places. the molecule trifluoromethylsulfur pentafluoride is one such tracer. draw an electron dot structure for cf3sf5, and predict the bond angles around both carbon and sulfur. 383. Therefore, tetrahedrals have a bond angle of 109.5 degrees. how scientists got that number was through experiments, but we don't need to know too much detail because that is not described in the textbook or lecture. using the example above, we would add that h 2 o has a bond angle of 109.5° and co 2 would have a bond angle of 180°. Electron geometry: describes the arrangement of bonds and lone pairs around a central atom. molecular geometry: describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons. hybridization: orbitals are combined in order to spread out electrons. bond angles: the angle between adjacent bonds of an atom.

Ideal Bond Angles вђ Overview Examples Expii Therefore, tetrahedrals have a bond angle of 109.5 degrees. how scientists got that number was through experiments, but we don't need to know too much detail because that is not described in the textbook or lecture. using the example above, we would add that h 2 o has a bond angle of 109.5° and co 2 would have a bond angle of 180°. Electron geometry: describes the arrangement of bonds and lone pairs around a central atom. molecular geometry: describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons. hybridization: orbitals are combined in order to spread out electrons. bond angles: the angle between adjacent bonds of an atom.

Deviation From Ideal Bond Angles вђ Overview Examples Expii

Comments are closed.