Ib Chemistry Topic 4 4 2 Covalent Bonding Teaching Resourc

Ib Chemistry Topic 4 4 2 Covalent Bonding Teachin Ib chemistry topic 4 4.2 covalent bonding presentation . this website and its content is subject to our terms and conditions. Topic 4 chemical bonding and structure. this is a compilation of several videos covering all areas of chemical bonding and structure. the videos average out to be around 5 minutes long, so it is helpful to those who have a short attention span but want to cover all aspects of the material. it is recommended that you take notes or highlight in.
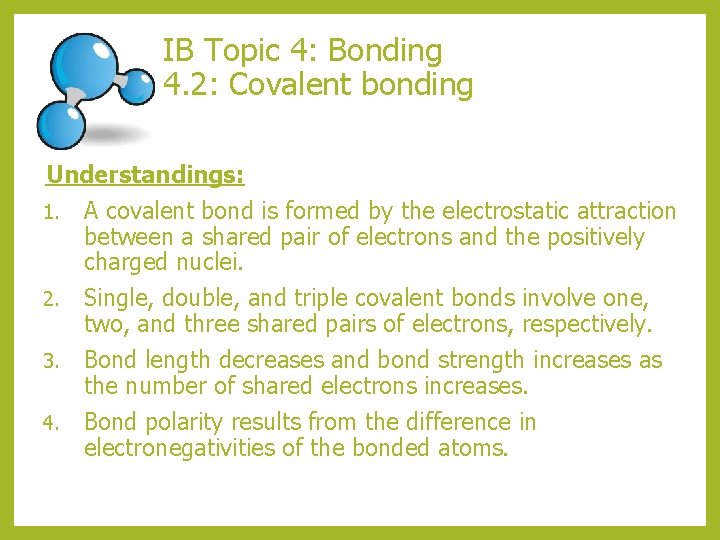
Topic 4 Bonding 4 2 Covalent Bonding Topic Topic 4 bonding. 4.1 the octet rule. the “octet rule” refers to the tendency of atoms to gain a valence shell with a total of 8 electrons. some atoms, like be and b, might form stable compounds with incomplete octets of electrons. 4.1 electronegativity and bonding. this video covers the relationship between the difference in. With each f atom having 1 bond pair and three lone pairs (non bonding) so, each atom needs to pair up one unpaired electron, forming 1 bond. #2 h2o. hydrogen atoms have 1 valence electron. (1 h) oxygen atoms have 6. (8 o = 2.6) so paired up we get a molecule shown right. with the o atom having 2 bond pairs and 2 lone pairs (non bonding). In ionic, atoms lose or gain electrons. in covalent, atoms share electrons to achieve noble gas configuration. occurs between non metals. there are single bonds (f 2), double bonds (o 2) and triple bonds (n 2) there is also hf for example in which h does not acquire a octet rule. Flipped learning video on covalent bonding. this is designed as a teaching resource to be viewed as homework, so students can then bring questions to class f.
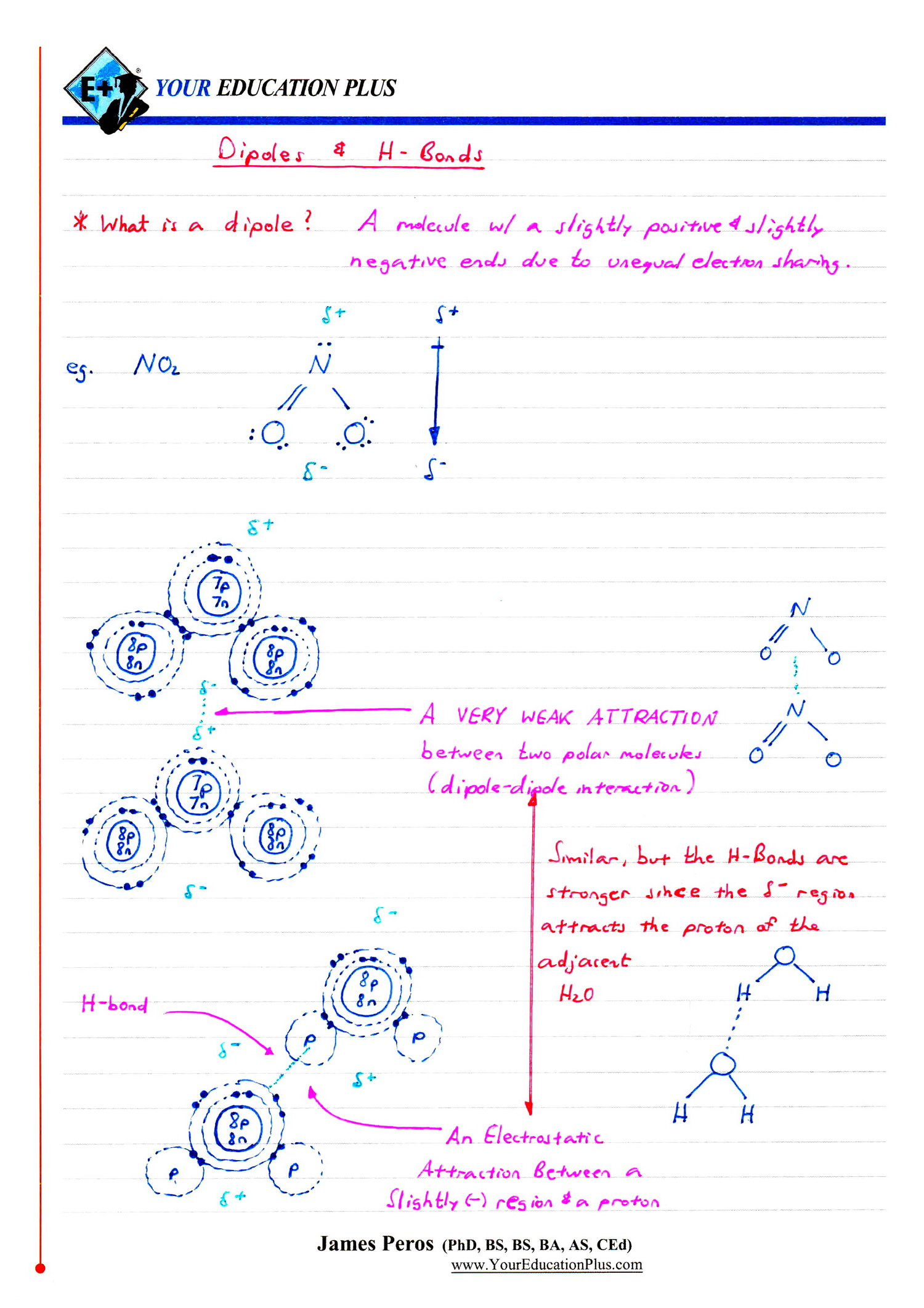
Ib Chemistry Sl Hl 4 2 Covalent Bonding In ionic, atoms lose or gain electrons. in covalent, atoms share electrons to achieve noble gas configuration. occurs between non metals. there are single bonds (f 2), double bonds (o 2) and triple bonds (n 2) there is also hf for example in which h does not acquire a octet rule. Flipped learning video on covalent bonding. this is designed as a teaching resource to be viewed as homework, so students can then bring questions to class f. Topic 4. chemical bonding and structure. basics. intramolecular bonds are strong chemical bonds. molecules are chemical species that are covalently bonded and have the structure of discrete molecules. compounds are 2 or more different atoms bonded together. melting boiling point rankings. Ib chemistry topic 4.2 covalent bondinghow to determine if a compound is covalent or ionic (degree of covalent character), how polar the covalent bond is and.
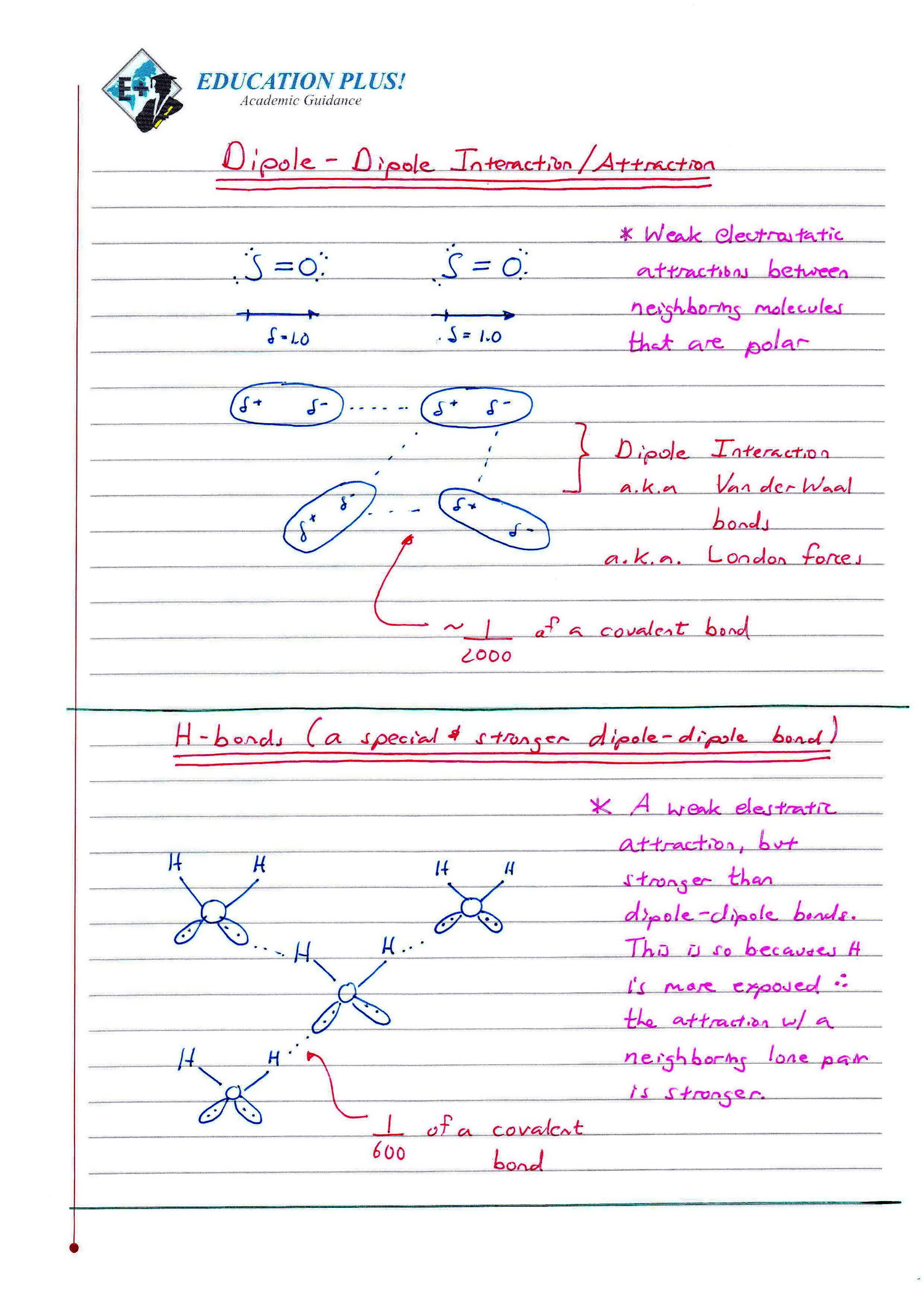
Ib Chemistry Sl Hl 4 2 Covalent Bonding Topic 4. chemical bonding and structure. basics. intramolecular bonds are strong chemical bonds. molecules are chemical species that are covalently bonded and have the structure of discrete molecules. compounds are 2 or more different atoms bonded together. melting boiling point rankings. Ib chemistry topic 4.2 covalent bondinghow to determine if a compound is covalent or ionic (degree of covalent character), how polar the covalent bond is and.

Comments are closed.