Ib Chemistry Sl Hl 4 2 Covalent Bonding
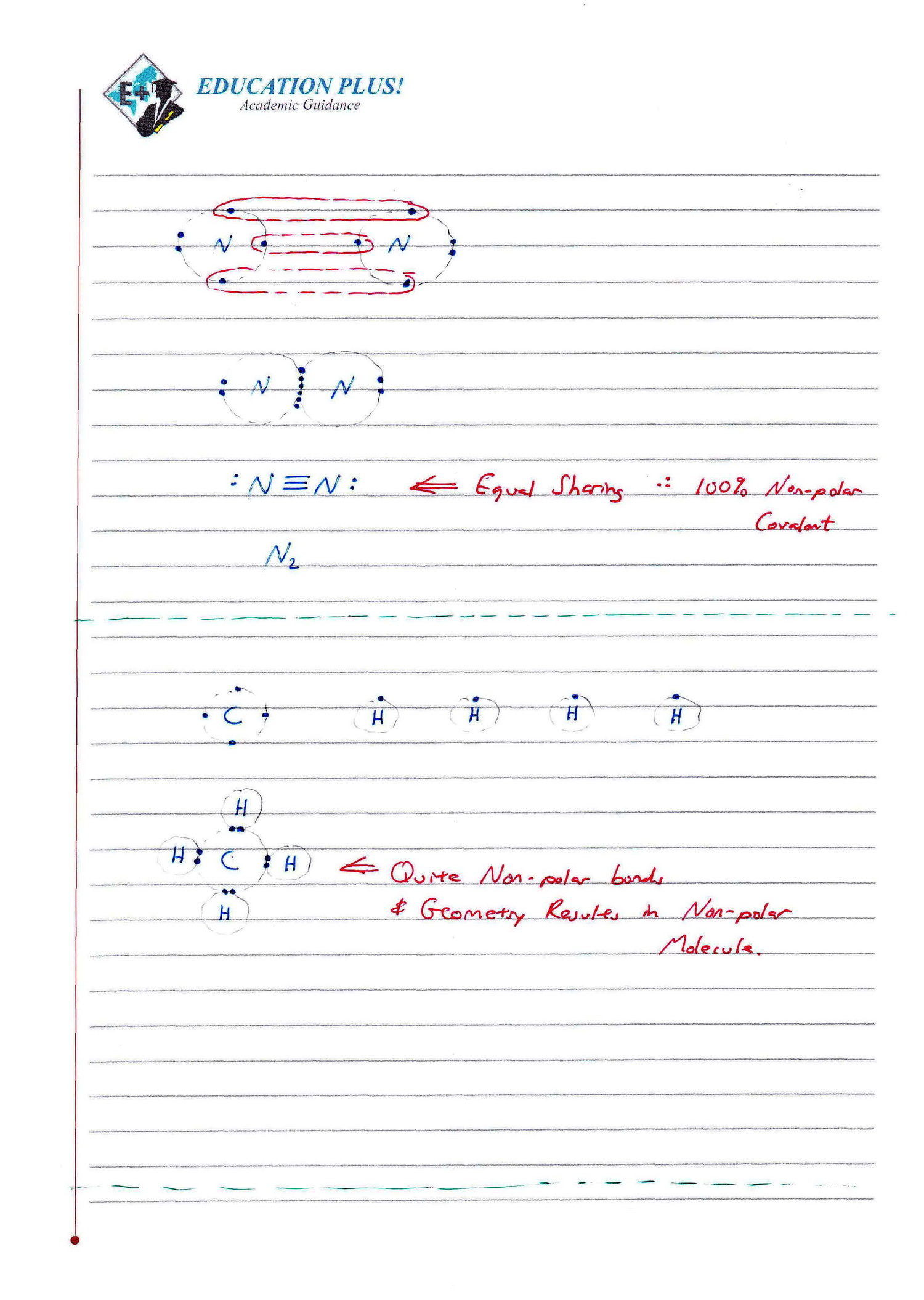
Ib Chemistry Sl Hl 4 2 Covalent Bonding In ionic, atoms lose or gain electrons. in covalent, atoms share electrons to achieve noble gas configuration. occurs between non metals. there are single bonds (f 2), double bonds (o 2) and triple bonds (n 2) there is also hf for example in which h does not acquire a octet rule. Revision villagethis video covers an overview of covalent bonding. part of the ib chemistry sl & hl course in structure 2.the content of this video provides.
Ib Chemistry Sl Hl 4 2 Covalent Bonding Flipped learning video on covalent bonding. this is designed as a teaching resource to be viewed as homework, so students can then bring questions to class f. Topic 4 bonding. 4.1 the octet rule. the “octet rule” refers to the tendency of atoms to gain a valence shell with a total of 8 electrons. some atoms, like be and b, might form stable compounds with incomplete octets of electrons. 4.1 electronegativity and bonding. this video covers the relationship between the difference in. Topic 4. chemical bonding and structure. basics. intramolecular bonds are strong chemical bonds. molecules are chemical species that are covalently bonded and have the structure of discrete molecules. compounds are 2 or more different atoms bonded together. melting boiling point rankings. With each f atom having 1 bond pair and three lone pairs (non bonding) so, each atom needs to pair up one unpaired electron, forming 1 bond. #2 h2o. hydrogen atoms have 1 valence electron. (1 h) oxygen atoms have 6. (8 o = 2.6) so paired up we get a molecule shown right. with the o atom having 2 bond pairs and 2 lone pairs (non bonding).

Notes 4 2 Covalent Bond Formation Of Covalent Bond And Lewis Topic 4. chemical bonding and structure. basics. intramolecular bonds are strong chemical bonds. molecules are chemical species that are covalently bonded and have the structure of discrete molecules. compounds are 2 or more different atoms bonded together. melting boiling point rankings. With each f atom having 1 bond pair and three lone pairs (non bonding) so, each atom needs to pair up one unpaired electron, forming 1 bond. #2 h2o. hydrogen atoms have 1 valence electron. (1 h) oxygen atoms have 6. (8 o = 2.6) so paired up we get a molecule shown right. with the o atom having 2 bond pairs and 2 lone pairs (non bonding). Past papers. cie. spanish language & literature. past papers. other subjects. accounting. revision notes on 4.1.4 covalent bonds for the dp ib chemistry: hl syllabus, written by the chemistry experts at save my exams. Topic 8: acids and bases—6.5 hours for sl and hl. notes on acids and bases. 8.1: theory of acids and bases notes. 8.2: properties of acids and bases notes. 8.3: the ph scale notes. 8.4: strong and weak acids and bases notes. 8.5: acid deposition notes. general overview of acids and bases.

Comments are closed.