Ib Biology Topic 2 1 2 2 Molecules Water

Ib Biology Topic 2 1 2 2 Molecules Water Youtu This video covers ib biology subtopics 2.1 & 2.2 from the ib biology topic 2 syllabus content.this ib biology video begins by discussing the main classes of. 2.2 water. 2.2.u1 water molecules are polar and hydrogen bonds form between them. describe the structure of an atom (in terms of protons, neutrons and electrons). contrast ion with atom. define anion and cation. contrast covalent, ionic and hydrogen bonds. write the molecular formula for water and draw the atomic structure of the molecule.
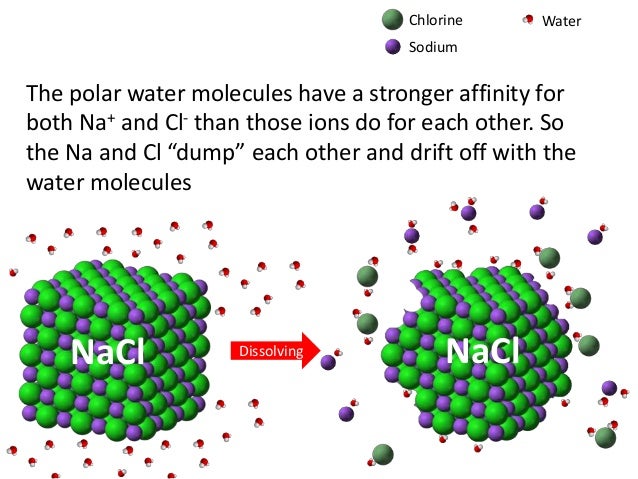
Ib Biology 2 2 Slides Water A1.1.4— adhesion of water to materials that are polar or charged and impacts for organisms. capillary action helps bring water from the roots all the way up to the branches and leaves. adhesion of water to the xylem walls will cause an upward force on the water. outline the cause and effect of capillary action in soil. Spanish. past papers. cie. spanish language & literature. past papers. other subjects. accounting. revision notes on 2.1.1 molecules for the dp ib biology: sl syllabus, written by the biology experts at save my exams. A1.1.1 water as medium of life. state that the first cells originated in water. list reasons why water is a substance on which life depends . a1.1.2— hydrogen bonds as a consequence of the polar covalent bonds within water molecules. describe the structure of an atom. outline the formation of ionic and covalent bonds between atoms. Essential idea: water is the medium of life. 2.2.u1 water molecules are polar and hydrogen bonds form between them. describe the structure of an atom (in terms of protons, neutrons and electrons). contrast ion with atom. define anion and cation. contrast covalent, ionic and hydrogen bonds.

Topic 2 Molecular Biology 2 2 Water Ib A1.1.1 water as medium of life. state that the first cells originated in water. list reasons why water is a substance on which life depends . a1.1.2— hydrogen bonds as a consequence of the polar covalent bonds within water molecules. describe the structure of an atom. outline the formation of ionic and covalent bonds between atoms. Essential idea: water is the medium of life. 2.2.u1 water molecules are polar and hydrogen bonds form between them. describe the structure of an atom (in terms of protons, neutrons and electrons). contrast ion with atom. define anion and cation. contrast covalent, ionic and hydrogen bonds. 2. 2.2.1 water molecules are polar and hydrogen bonds form between them. • water (h2o) is made up of two hydrogen atoms covalently bound to an oxygen atom • while this bonding involves the sharing of electrons, they are not shared equally • the number of protons in each atom is different; oxygen atoms have 8 whilst hydrogen atoms have just 1 • having more protons the oxygen atoms. Topic 2. 2 water ib biology resources. ib biology resources. menu. 2.2.u1 water molecules are polar and hydrogen bonds form between them. 2.2.u2 hydrogen bonding and dipolarity explain the cohesive, adhesive, thermal and solvent properties of water. [students should know at least one example of a benefit to living organisms of each property.

Ib Diploma Biology Topic 2 2 Water Teaching Resources 2. 2.2.1 water molecules are polar and hydrogen bonds form between them. • water (h2o) is made up of two hydrogen atoms covalently bound to an oxygen atom • while this bonding involves the sharing of electrons, they are not shared equally • the number of protons in each atom is different; oxygen atoms have 8 whilst hydrogen atoms have just 1 • having more protons the oxygen atoms. Topic 2. 2 water ib biology resources. ib biology resources. menu. 2.2.u1 water molecules are polar and hydrogen bonds form between them. 2.2.u2 hydrogen bonding and dipolarity explain the cohesive, adhesive, thermal and solvent properties of water. [students should know at least one example of a benefit to living organisms of each property.

Ib Diploma Biology Topic 2 2 Water Teaching Resources

Comments are closed.