Homogeneous And Homogeneous Mixtures
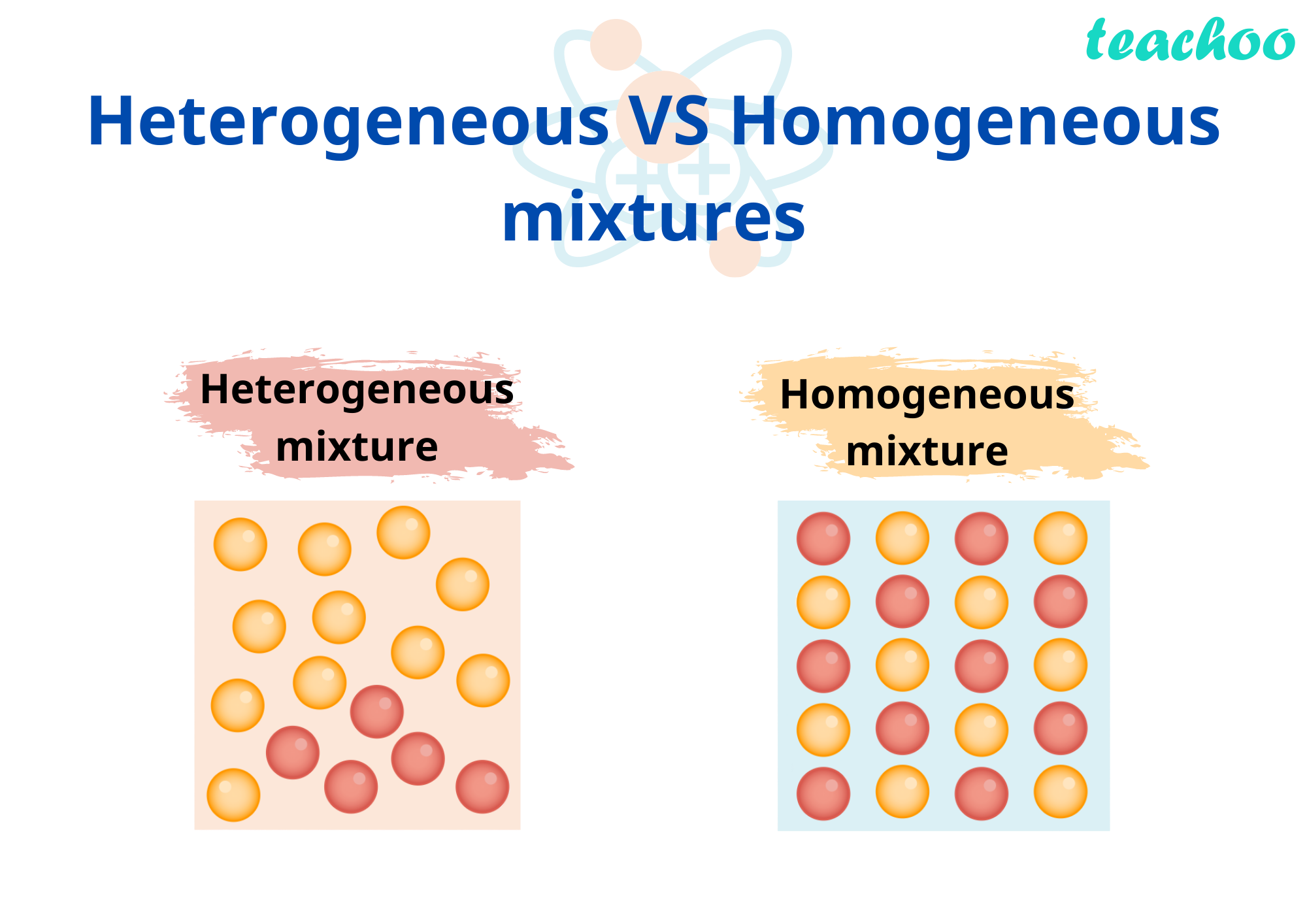
Homogeneous And Homogeneous Mixtures A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. on the other hand, a heterogeneous mixture is a mixture in which the components of the mixture are not uniform or have localized regions with different properties. learn more about the difference between these. A homogeneous mixture is a single phase with a uniform composition of multiple components. learn how to distinguish homogeneous mixtures from heterogeneous mixtures and pure substances, and see examples of homogeneous mixtures in chemistry and everyday life.

What Is A Mixture In Chemistry The Chemistry Blog A homogeneous mixture appears uniform, regardless of where you sample it. a heterogeneous mixture contains particles of different shapes or sizes, and the composition of one sample may differ from that of another sample. whether a mixture is heterogeneous or homogeneous depends on how closely you examine it. sand may appear homogeneous from a. Saltwater: the combination of salt and water forms a homogeneous mixture where the salt molecules uniformly disperse in the water. air: despite being a mixture of gases like nitrogen, oxygen, and others, the air we breathe is homogeneous. properties. uniform appearance: homogeneous mixtures display a consistent appearance. Learn the definitions, examples and differences of homogeneous and heterogeneous mixtures in chemistry. homogeneous mixtures have a uniform composition and only one phase, while heterogeneous mixtures have a non uniform composition and two or more phases. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how they differ in composition, state of matter, and properties. find out how some mixtures can switch between homogeneous and heterogeneous under certain conditions.

Comments are closed.