Heme Synthesis Synthesis Of Porphyrin Molecule Vrogue Co
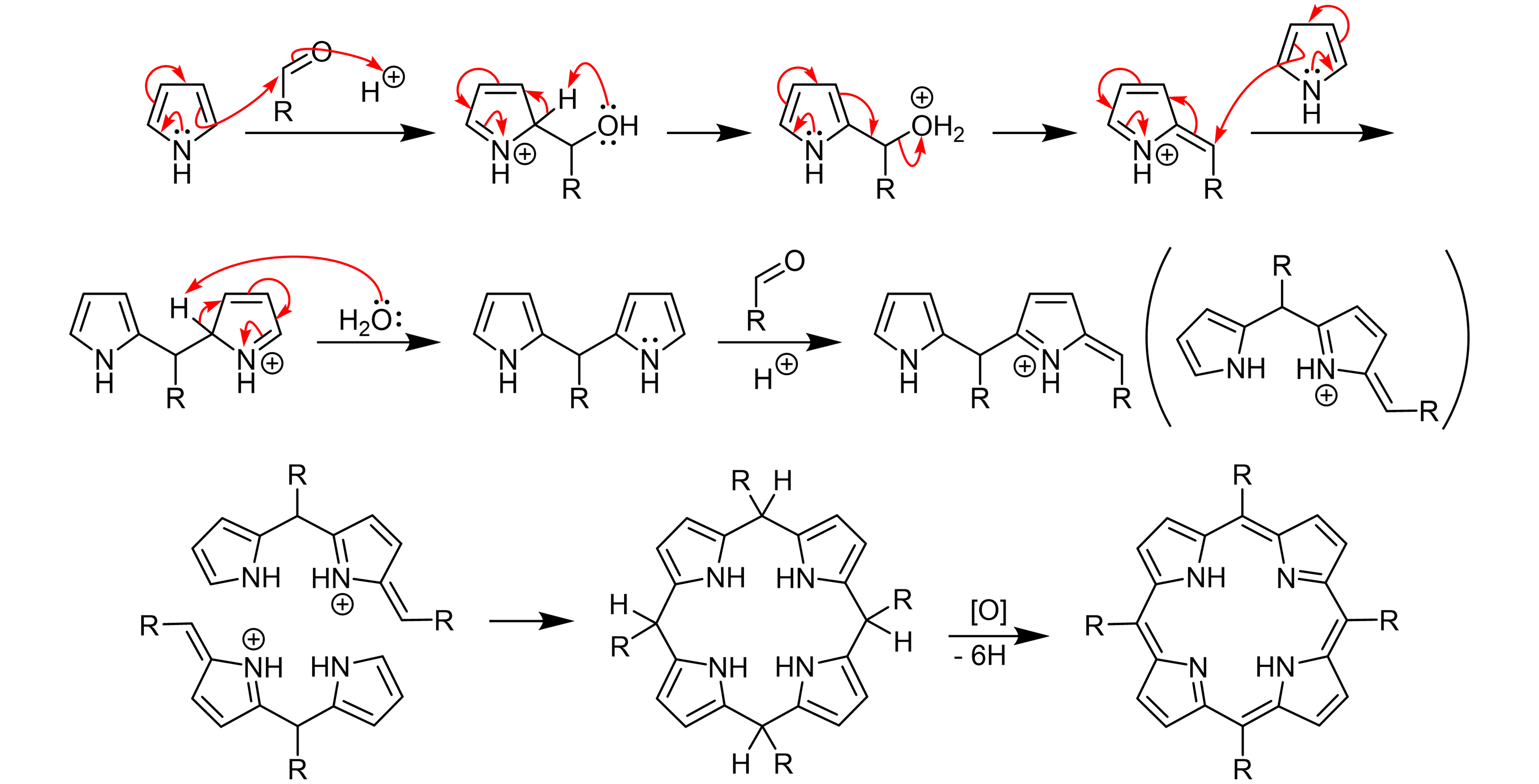
Heme Synthesis Synthesis Of Porphyrin Molecule Vrogue Co Cellular level. porphyrin synthesis is the process that produces heme. heme synthesis occurs partly in the mitochondria and partly in the cytosol. the biosynthesis involves an eight step enzymatic pathway. heme biosynthesis starts in mitochondria with the condensation of succinyl co a from the citric acid cycle and an amino acid glycine. Heme is a term used for ferrous protoporphyrin ix (ppix) that is easily oxidized in vitro to hemin, termed ferric ppix. in heme the ferrous iron atom (fe 2 ) has 6 electron pairs, 4 are bound to the pyrrolic nitrogens of the porphyrin macrocyle, leaving two unoccupied electron pairs, one above and the other below the plane of the porphyrin.
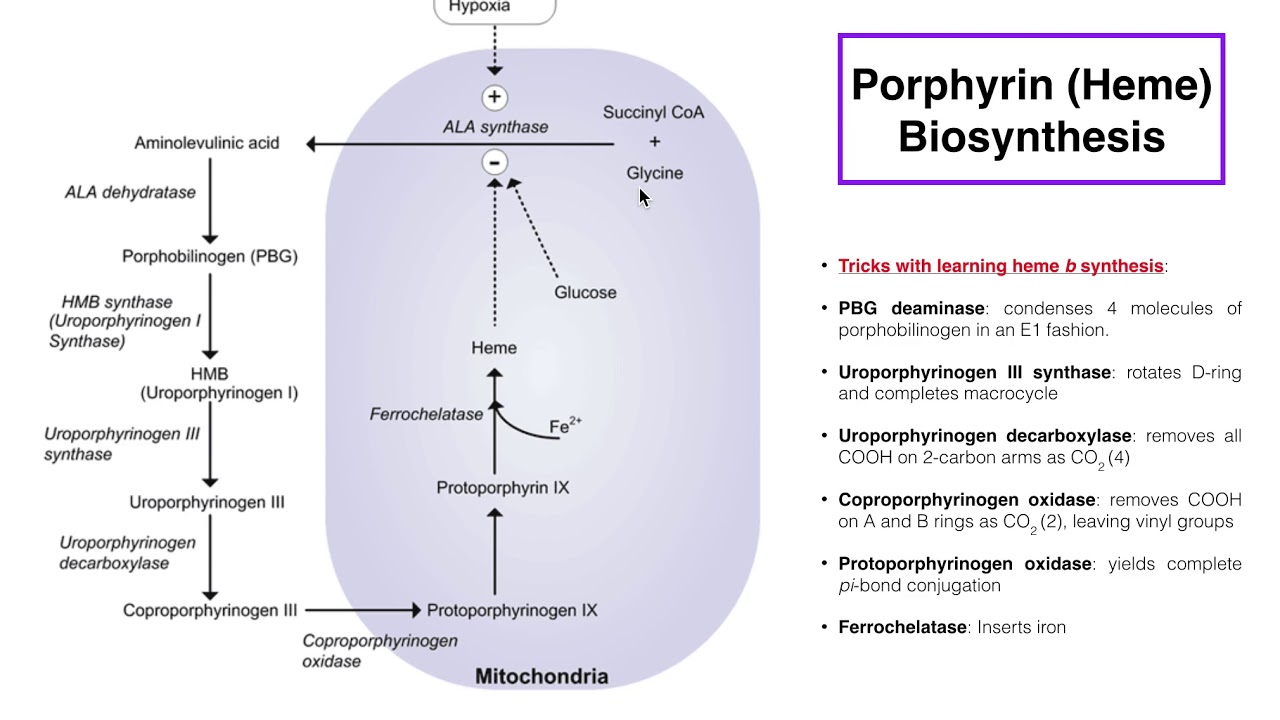
Heme Synthesis Synthesis Of Porphyrin Molecule Vrogue Co The reactions involved in heme synthesis are as follows: 1. condensation of glycine and succinyl coa. in the first step of heme synthesis, the enzyme ala synthase catalyzes the condensation reaction between glycine and succinyl coa. this results in the formation of δ aminolevulinic acid (ala). this reaction, catalyzed by the enzyme ala. Heme (iron protoporphyrin ix) is an essential molecule for numerous living organisms. not only does it serve as a prosthetic group in enzymes, it also acts as a signaling molecule that controls. The biosynthesis of heme b, c and a, and all other naturally occurring tetrapyrroles starts with the formation of the common precursor molecule 5 aminolevulinic acid (ala). depending on the organism, ala is made by one of two unrelated biosynthetic routes, the so called shemin or c4 pathway and the c 5 pathway. Another landmark chlorin total synthesis of the modern era is battersby's enantioselective pathway to the heme d 1 methyl ester 237. 112 heme d 1 is one of the two heme units found within the bacterial enzyme cytochrome cd 1. 112 although its gross chemical structure was elucidated in the mid 1980s, as a result of chang and wu's synthesis of a.

Comments are closed.