Heating Matter And Changes In State
Changes In States Of Matter Good Science What you will learn. in this lesson, you will learn how heating can change the state of matter! you will discover that when ice (a solid) gets warm, it melts into water (a liquid), and when water gets even hotter, it turns into water vapor (a gas). you will also understand how the tiny particles in solids, liquids, and gases behave differently. #matter #states #ngscience ngscience observe how the particle behave and the change in state that occurs when matter is heated. head to ngscience.c.

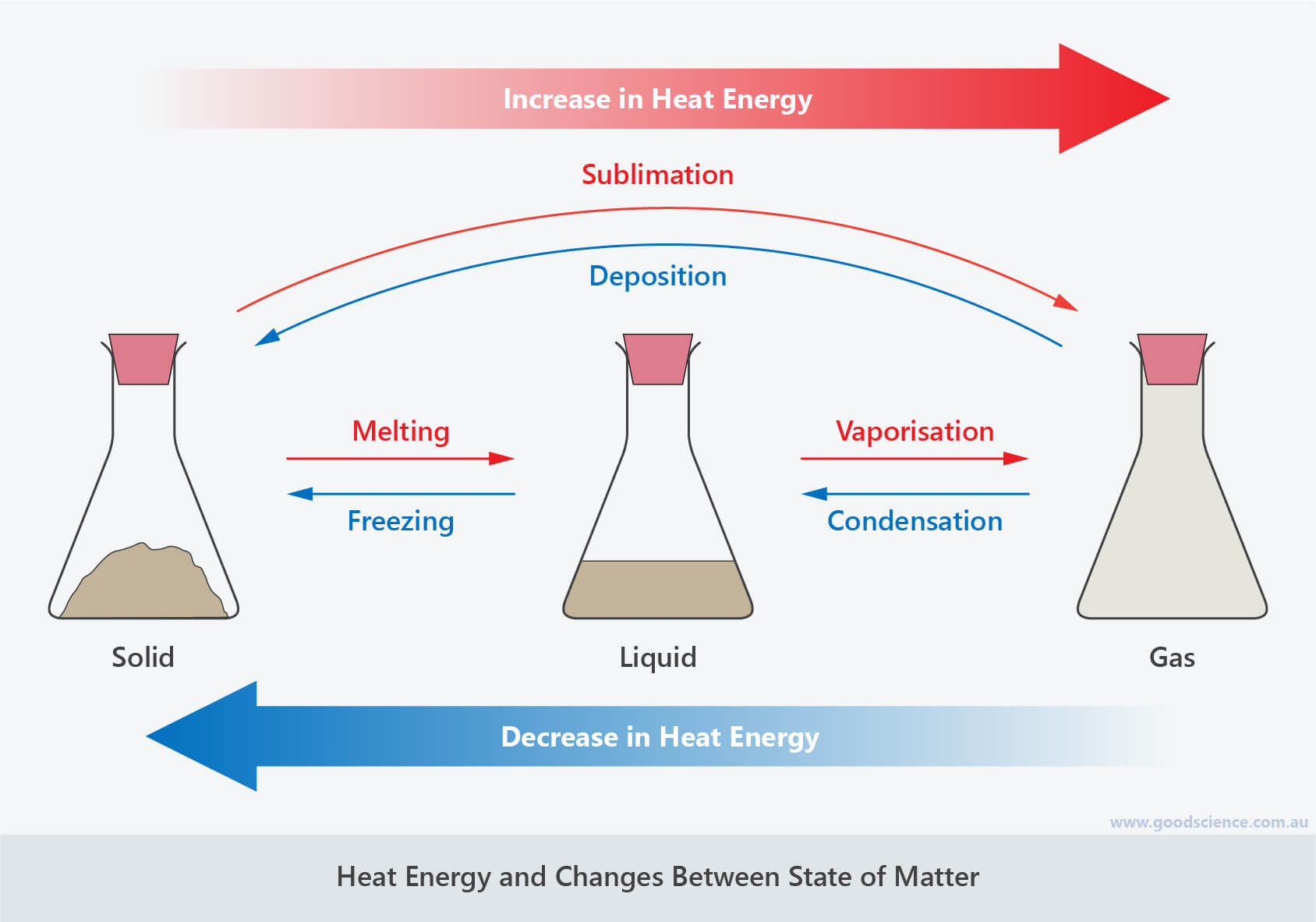
Heating Matter And Changes In State Youtube The temperature reflects the thermal energy content of the material—the addition of heat increase the vibrational motions, and temperature increases. ultimately, the solid changes to a liquid and the liquid changes to a gas phase as more heat is added, as illustrated in figure 1.9.1. figure 1.9.1: illustration of the relationship between. The energy change associated with each common phase change is shown in figure 2.5.1 2.5. 1. Δ h is positive for any transition from a more ordered to a less ordered state and negative for a transition from a less ordered to a more ordered state. previously, we defined the enthalpy changes associated with various chemical and physical processes. Heating a substance in the solid state will cause it to melt close melting the process that occurs when a solid turns into a liquid when it is heated., which changes it to the liquid state. You need it in a liquid state. similarly, other compounds are more useful in a particular state. the important part of state changes is the amount of energy that must be added or taken out to change the state. the temperature of a phase change remains constant while the energy is exchanged. only when all of the compound is in a particular state.

Heat And Change Of State вђ Science Learning Hub Heating a substance in the solid state will cause it to melt close melting the process that occurs when a solid turns into a liquid when it is heated., which changes it to the liquid state. You need it in a liquid state. similarly, other compounds are more useful in a particular state. the important part of state changes is the amount of energy that must be added or taken out to change the state. the temperature of a phase change remains constant while the energy is exchanged. only when all of the compound is in a particular state. Conversely, any transition from a less ordered to a more ordered state (liquid to solid, gas to liquid, or gas to solid) releases energy; it is exothermic. the energy change associated with each common phase change is shown in figure 11.5.1. in chapter 9, we defined the enthalpy changes associated with various chemical and physical processes. There are multiple different ways of modeling changes in state mathematically. the most common form is using the equations. q = m ⋅ c ⋅ Δ t and q = m ⋅ h (heat of fusion vaporization) m = mass of substance n = moles of substance c = specific heat of substance. as shown in the heating curve above, these equations are used interchangeably.

Comments are closed.