Heating Curves Phase Diagrams Mr Pauller Youtube

Heating Curves Phase Diagrams Mr Pauller Youtube This video discusses the heating curve and phase diagram for water. What is a phase diagram? fully explained phase diagrams. fully explained heating curves of any substance.

Heating And Cooling Curves Explained The basics of the phase diagram and the heating curve are explained, including in the latter case, the concept of latent heat. these are two different types. Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. The energy change associated with each common phase change is shown in figure 2.5.1 2.5. 1. Δ h is positive for any transition from a more ordered to a less ordered state and negative for a transition from a less ordered to a more ordered state. previously, we defined the enthalpy changes associated with various chemical and physical processes. Phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase diagram depicts the phase.
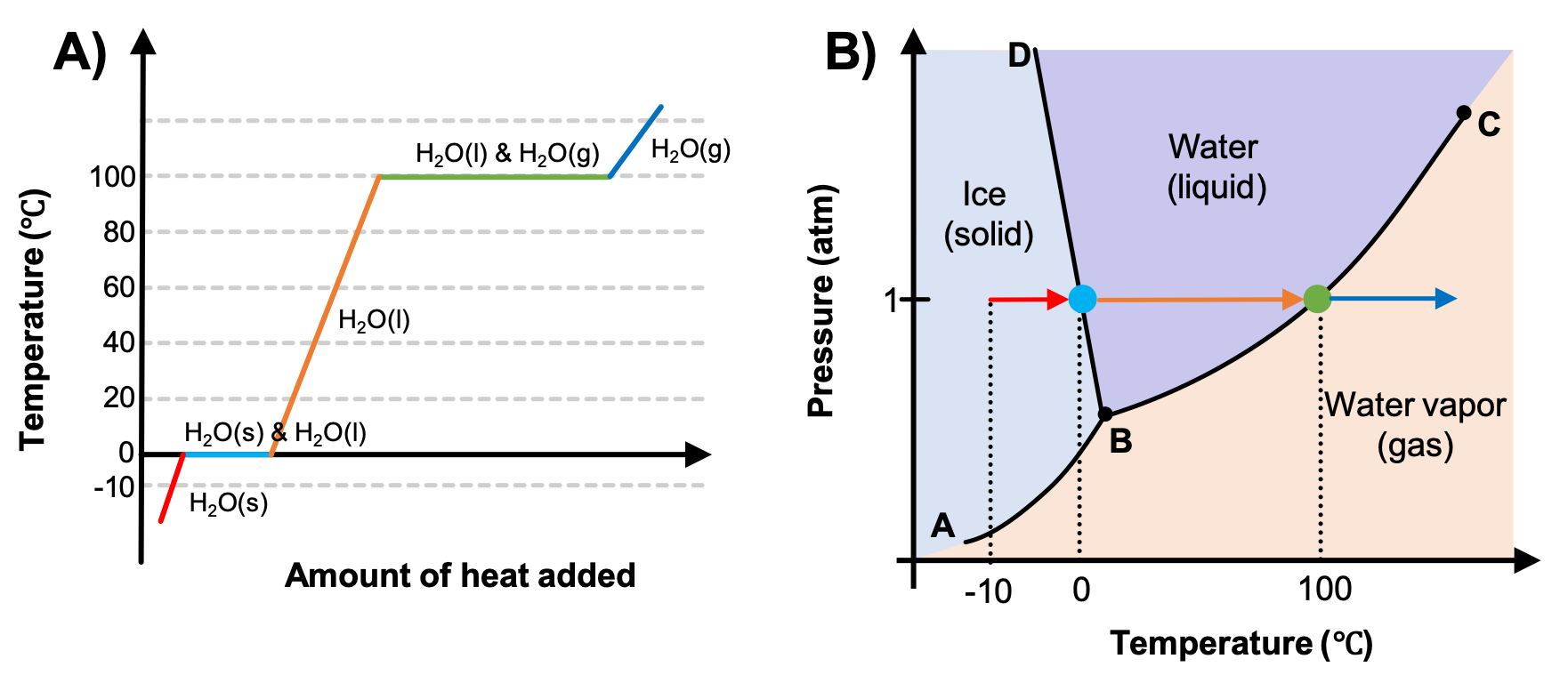
M11q2 Heating Curves And Phase Diagrams вђ Chem 103 104 Resource Book The energy change associated with each common phase change is shown in figure 2.5.1 2.5. 1. Δ h is positive for any transition from a more ordered to a less ordered state and negative for a transition from a less ordered to a more ordered state. previously, we defined the enthalpy changes associated with various chemical and physical processes. Phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase diagram depicts the phase. The experimental set up we imagined would generate a heating curve. heating and cooling curves are graphs. they plot a substance's temperature (y axis) against heat (x axis). for heating curves, we start with a solid and add heat energy. for cooling curves, we start with the gas phase and remove heat energy. cooling and heating curves have five. Heating curves. recall the relationship between the amount of heat absorbed or released by a substance, q, and its accompanying temperature change, Δt, already introduced in this module: q = m × c × Δt. where m is the mass of the substance and c is its specific heat. the relation applies to matter being heated or cooled, but not undergoing.

Comments are closed.