Heating And Cooling Curves вђ Overview Examples Expii

Heating And Cooling Curves Explained A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember. The region furthest to the left is solids, in the middle is liquids and, the right side is the gas phase. we can develop a phase change diagram for almost any element or molecule. for example, you could look up the phase change diagram for water or carbon dioxide. if you're interested in materials chemistry, they often use phase change diagrams.

Heating And Cooling Curves вђ Overview Examples Expii For water, that's 0oc. at equilibrium, both the water and ice are at zero degrees celsius. recall water's heating and cooling curve. at zero celsius, we have a plateau. that is the temperature we are breaking or reforming the hydrogen bonds. some of the liquid molecules have a low enough energy that they hydrogen bond to the ice. but, some of. Heating and cooling curves вђ overview examples expii boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Lesson summary. let's review. a heating or cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing temperature. the sloped areas.
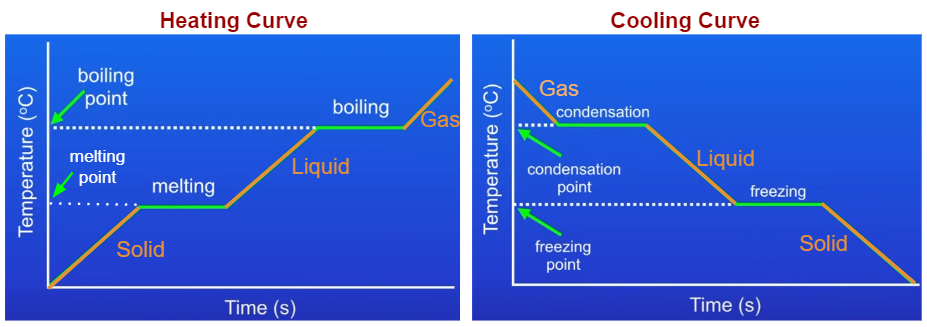
Heating And Cooling Graphs Examples Solutions Videos Notes The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Lesson summary. let's review. a heating or cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing temperature. the sloped areas. Understanding heating and cooling curves is crucial for grasping how substances absorb or release heat during phase changes. as a substance heats up, it undergoes an endothermic process, indicated by a positive heat variable (q), absorbing energy to break molecular bonds and transition from solid to liquid (melting or fusion) and eventually to gas (vaporization). Short summary. a heating curve is a graphical representation of the relationship between the temperature and heat of a substance. it can be broken down into five stages:the temperature at which a.

Heating And Cooling Curve Chart Understanding heating and cooling curves is crucial for grasping how substances absorb or release heat during phase changes. as a substance heats up, it undergoes an endothermic process, indicated by a positive heat variable (q), absorbing energy to break molecular bonds and transition from solid to liquid (melting or fusion) and eventually to gas (vaporization). Short summary. a heating curve is a graphical representation of the relationship between the temperature and heat of a substance. it can be broken down into five stages:the temperature at which a.

Comments are closed.