Grignard Reaction Organic Chemistry Video Clutch Prep
Grignard Reaction Organic Chemistry Video Clutch Prep Synthesis of primary, secondary, and tertiary alcohols from aldehydes and ketones using grignard reagents. created by jay.watch the next lesson: www . Amine reactions. chad's video lectures cover a full year of organic chemistry broken up into 22 chapters. absolutely free and no sign up required!.
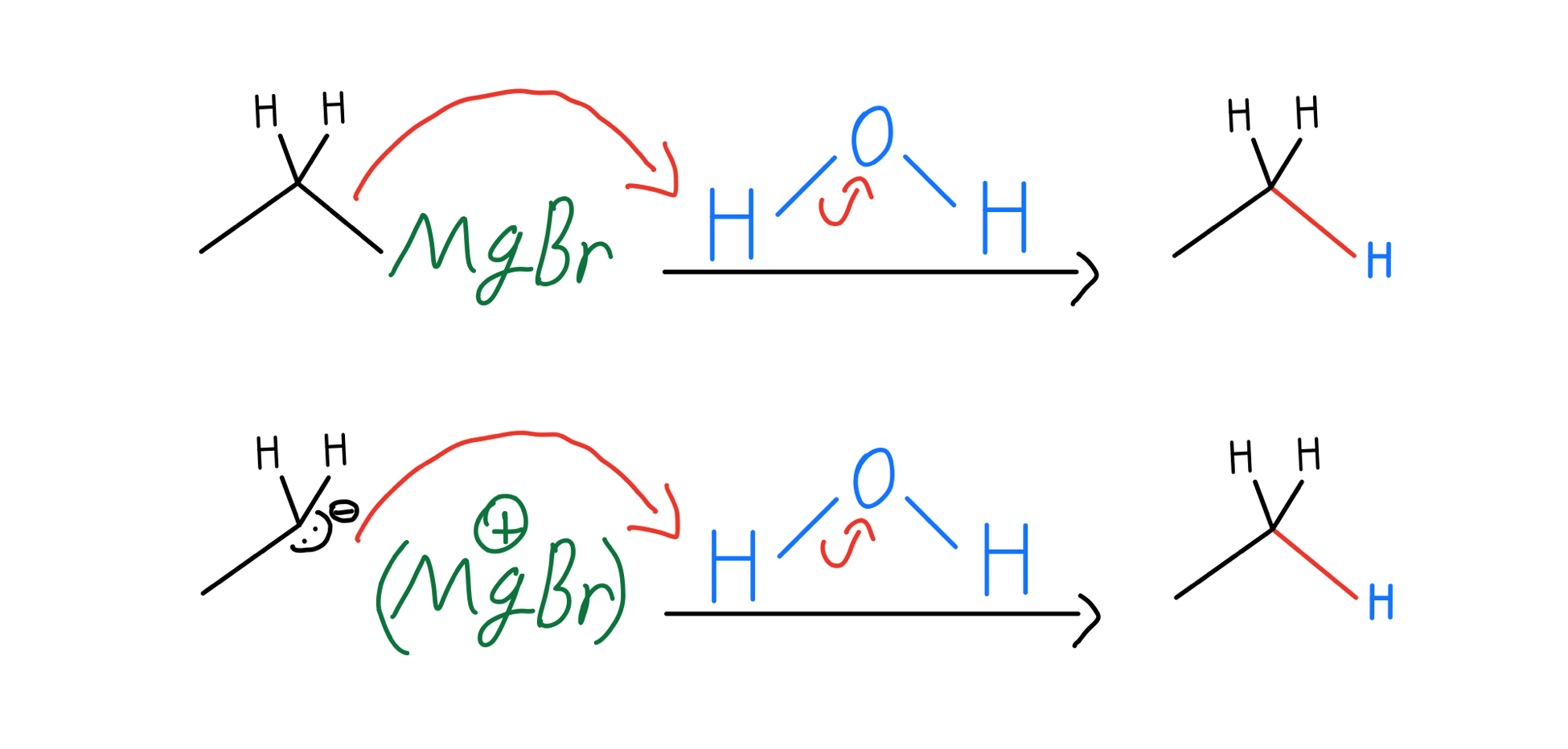
Grignard Reaction Organic Chemistry Video Clutch Prep The grignard reaction is a method for forming carbon carbon bonds between alkyl aryl halides and carbonyls like aldehydes, ketones, or esters. this nobel prize winning chemistry consists of two steps: grignard reagent formation and subsequent grignard addition onto a carbonyl to construct a new carbon carbon bond. Show how you would synthesize the following: (f) 2,5 dimethylhexane from a four carbon alkyl halide. predict the products of the following reactions. (a) allyl bromide cyclohexyl magnesium bromide. show how the reaction of an allylic halide with a grignard reagent might be used to synthesize the following h. 45 practice problem. a grignard reagent is formed when magnesium metal and 1 bromopent 2 ene are combined in dry ether. this grignard reagent produces a mixture of pent 1 ene, cis pent 2 ene, and trans pent 2 ene when water is added. adding water produces the same combination of products in the same ratios when the grignard reagent is. The grignard reaction is an incredibly versatile method of making new carbon carbon bonds. for many years, it was the premiere method of the carbon carbon bond formation. it’s made such a profound impact on the field of organic chemistry, that early 1900’s to about 1960 is known as the grignard era of organic chemistry.
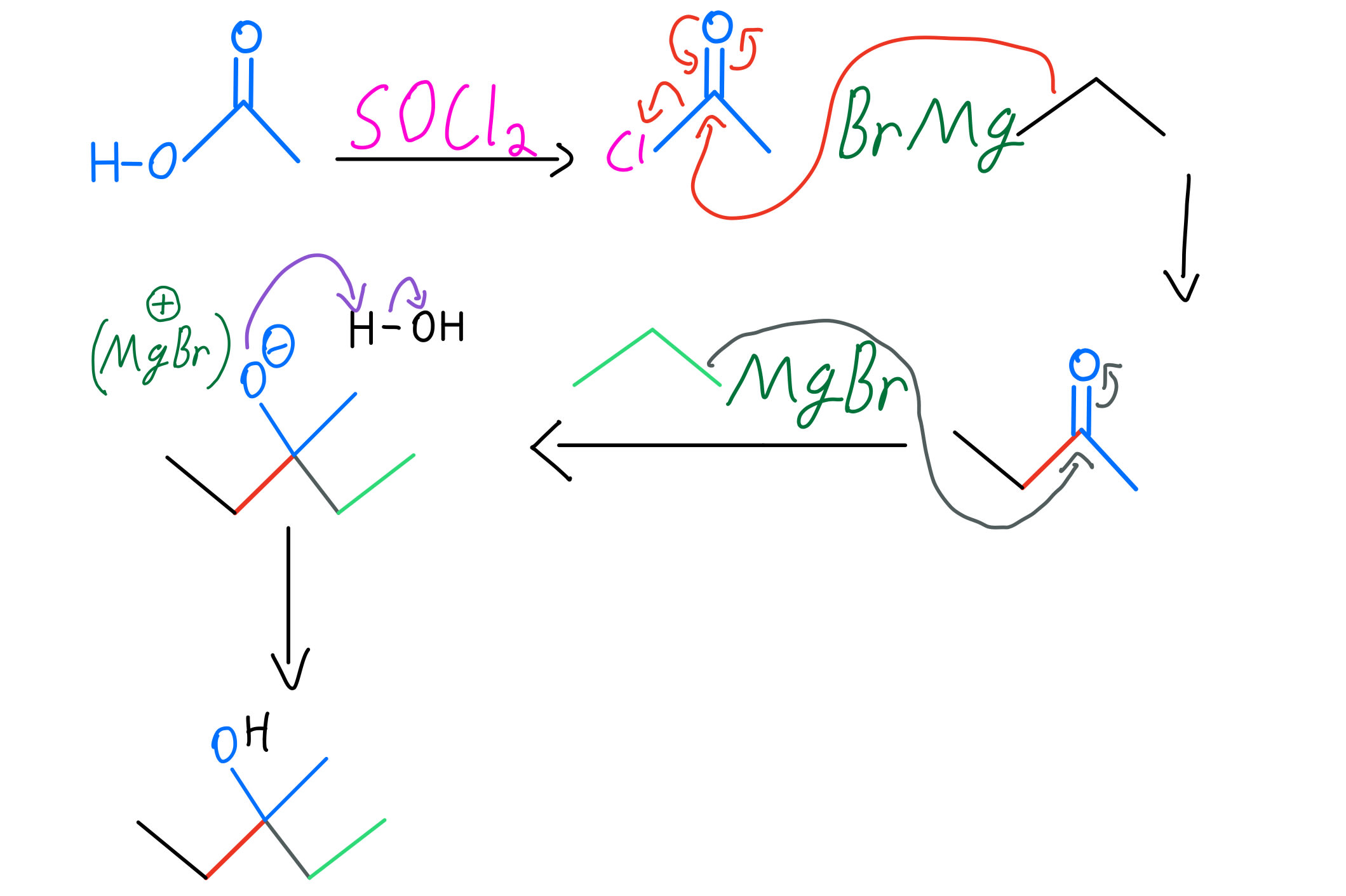
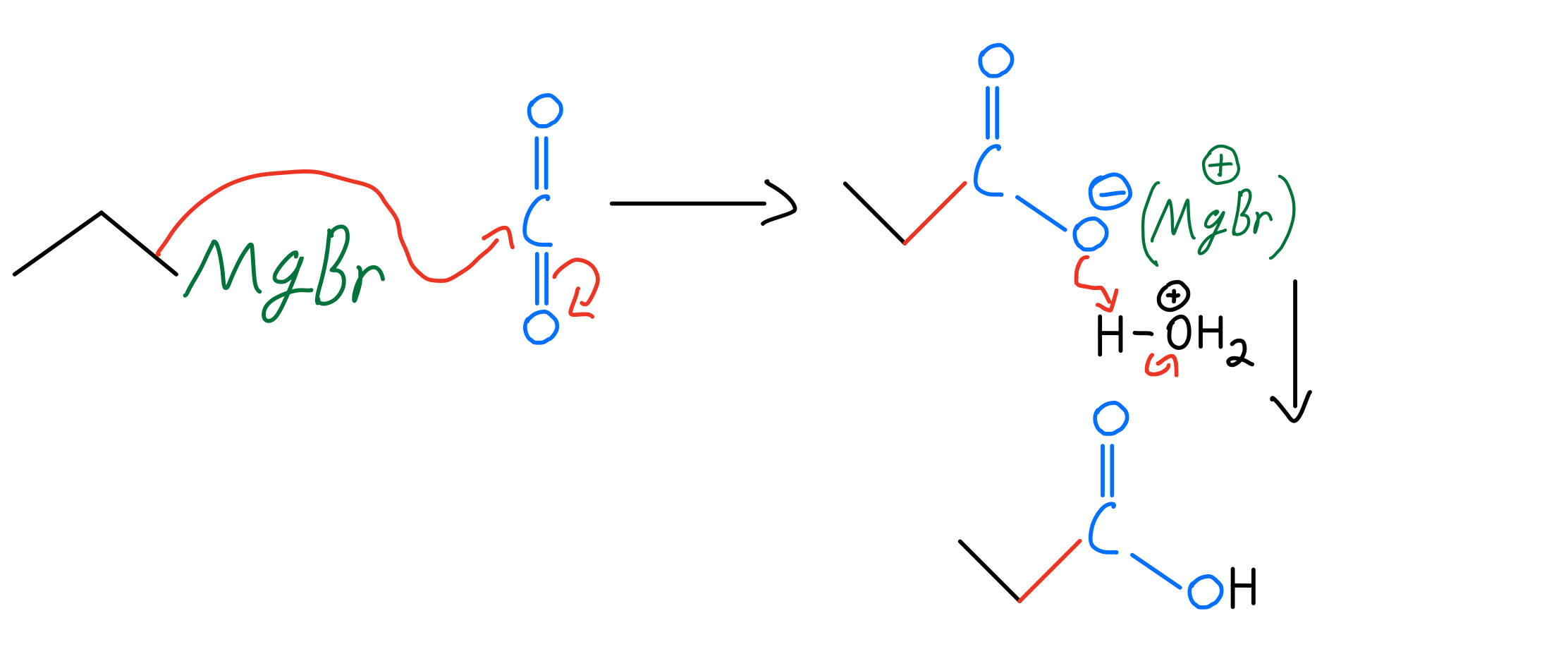
Grignard Reaction Organic Chemistry Video Clutch Prep 45 practice problem. a grignard reagent is formed when magnesium metal and 1 bromopent 2 ene are combined in dry ether. this grignard reagent produces a mixture of pent 1 ene, cis pent 2 ene, and trans pent 2 ene when water is added. adding water produces the same combination of products in the same ratios when the grignard reagent is. The grignard reaction is an incredibly versatile method of making new carbon carbon bonds. for many years, it was the premiere method of the carbon carbon bond formation. it’s made such a profound impact on the field of organic chemistry, that early 1900’s to about 1960 is known as the grignard era of organic chemistry. Grignard reagents are excellent carbon based nucleophiles as well as strong bases. they will add twice to esters to give tertiary alcohols. grignard reagents will also react with carbon dioxide (co 2) to give carboxylic acids (after acid workup). grignard reagents will not perform sn2 reactions with alkyl halides. Using a grignard reaction to synthesize an alcohol. how could you use the reaction of a grignard reagent with a carbonyl compound to synthesize 2 methyl 2 pentanol? strategy draw the product, and identify the three groups bonded to the alcohol carbon atom. if the three groups are all different, the starting carbonyl compound must be a ketone.

Grignard Reaction Organic Chemistry Video Clutch Prep Grignard reagents are excellent carbon based nucleophiles as well as strong bases. they will add twice to esters to give tertiary alcohols. grignard reagents will also react with carbon dioxide (co 2) to give carboxylic acids (after acid workup). grignard reagents will not perform sn2 reactions with alkyl halides. Using a grignard reaction to synthesize an alcohol. how could you use the reaction of a grignard reagent with a carbonyl compound to synthesize 2 methyl 2 pentanol? strategy draw the product, and identify the three groups bonded to the alcohol carbon atom. if the three groups are all different, the starting carbonyl compound must be a ketone.

Comments are closed.