Grignard Reaction
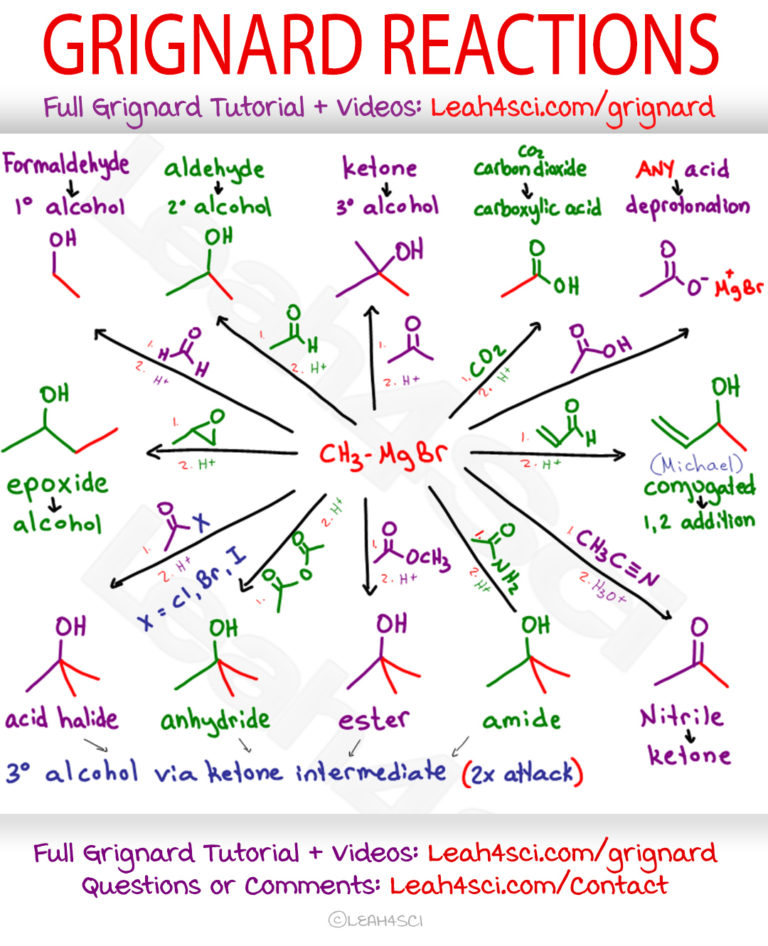
Grignard Reaction Mechanism Reagent And Cheat Sheet Learn about the organometallic reaction of grignard reagents with carbonyl compounds, named after the nobel laureate victor grignard. find out the history, mechanism, conditions, and variants of this important coupling reaction. Learn about the addition of organomagnesium halides (grignard reagents) to ketones, aldehydes, esters, nitriles and other compounds. find the mechanism, literature, examples and related reactions of grignard reagents.

Oberflг Che In Acht Nehmen Legitim Grignard Mechanism Krankenwagen Grignard reagents are excellent carbon based nucleophiles as well as strong bases. they will add twice to esters to give tertiary alcohols. grignard reagents will also react with carbon dioxide (co 2) to give carboxylic acids (after acid workup). grignard reagents will not perform sn2 reactions with alkyl halides. Learn about the grignard reaction, a key process in organic chemistry that uses organometallic compounds to form new carbon carbon bonds. find out the history, mechanism, importance, types, and examples of the grignard reaction and reagent. Learn how to make and use grignard reagents, which are alkyl or aryl magnesium compounds that react with water, carbon dioxide and carbonyl compounds. see examples of reactions with aldehydes, ketones and methanal to form alcohols. The grignard reaction, although useful, does have limitations. one major problem is that a grignard reagent can’t be prepared from an organohalide if other reactive functional groups are present in the same molecule. for example, a compound that is both an alkyl halide and a ketone can’t form a grignard reagent because it would react with.

Comments are closed.