Galvanic Cells Chemistry Steps
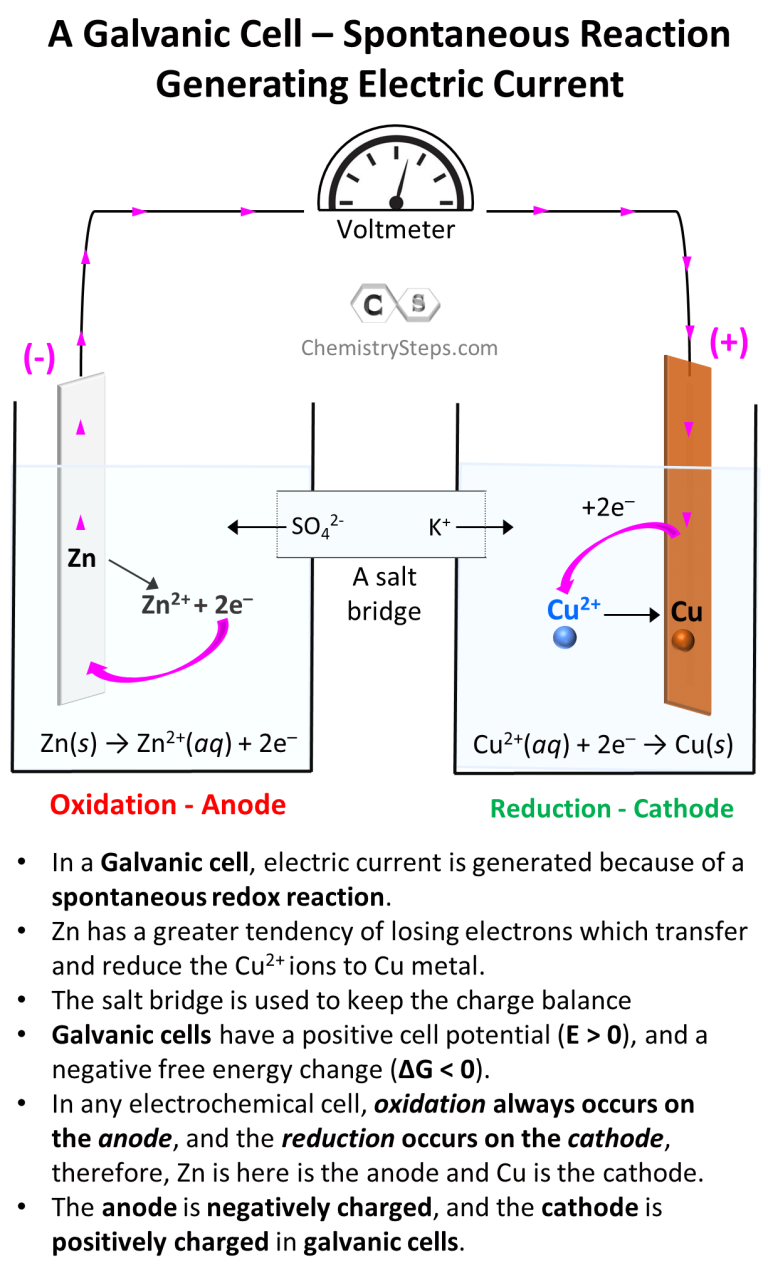
Galvanic Cells Chemistry Steps Galvanic cells have a positive cell potential (e > 0), and a negative free energy change (Δg < 0). in any electrochemical cell, oxidation always occurs on the anode, and the reduction occurs on the cathode, therefore, in our example, zn is the anode and cu is the cathode. the anode is negatively charged, and the cathode is positively charged. Galvanic or voltaic cells involve spontaneous electrochemical reactions in which the half reactions are separated (figure \(\pageindex{2}\)) so that current can flow through an external wire. the beaker on the left side of the figure is called a half cell, and contains a 1 m solution of copper(ii) nitrate [cu(no 3 ) 2 ] with a piece of copper.
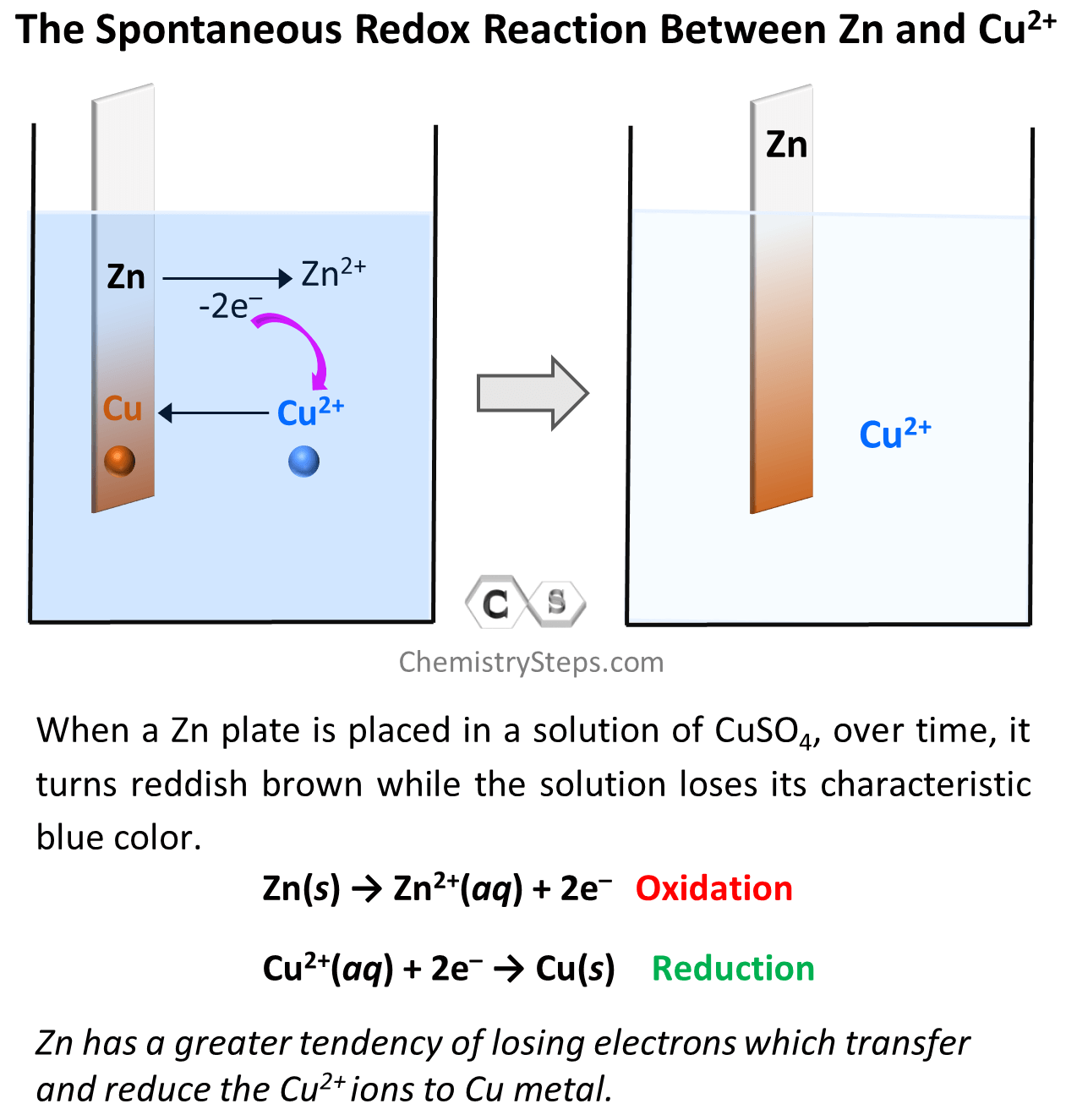
Galvanic Cells Chemistry Steps A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.2.2 17.2. 2. the cell is comprised of two half cells, each containing the redox conjugate pair (“couple”) of a single reactant. the half cell shown at the left contains the cu (0) cu (ii) couple in the form of a solid copper foil and an. Principle of galvanic (voltaic) cell. electric work done by a galvanic cell is mainly due to the gibbs energy of spontaneous redox reaction in the voltaic cell. it generally consists of two half cells and a salt bridge. each half cell further consists of a metallic electrode dipped into an electrolyte. these two half cells are connected to a. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. the cell is comprised of two half cells, each containing the redox conjugate pair (“couple”) of a single reactant. the half cell shown at the left contains the cu (0) cu (ii) couple in the form of a solid copper foil and an aqueous. The design of fuel cells requires extensive knowledge of electrochemistry. galvanic cells, also known as voltaic cells, are electrochemical cells in which spontaneous oxidation reduction reactions produce electrical energy. a battery is a galvanic cell. when two half reactions are separated so that electrical current can flow through an.
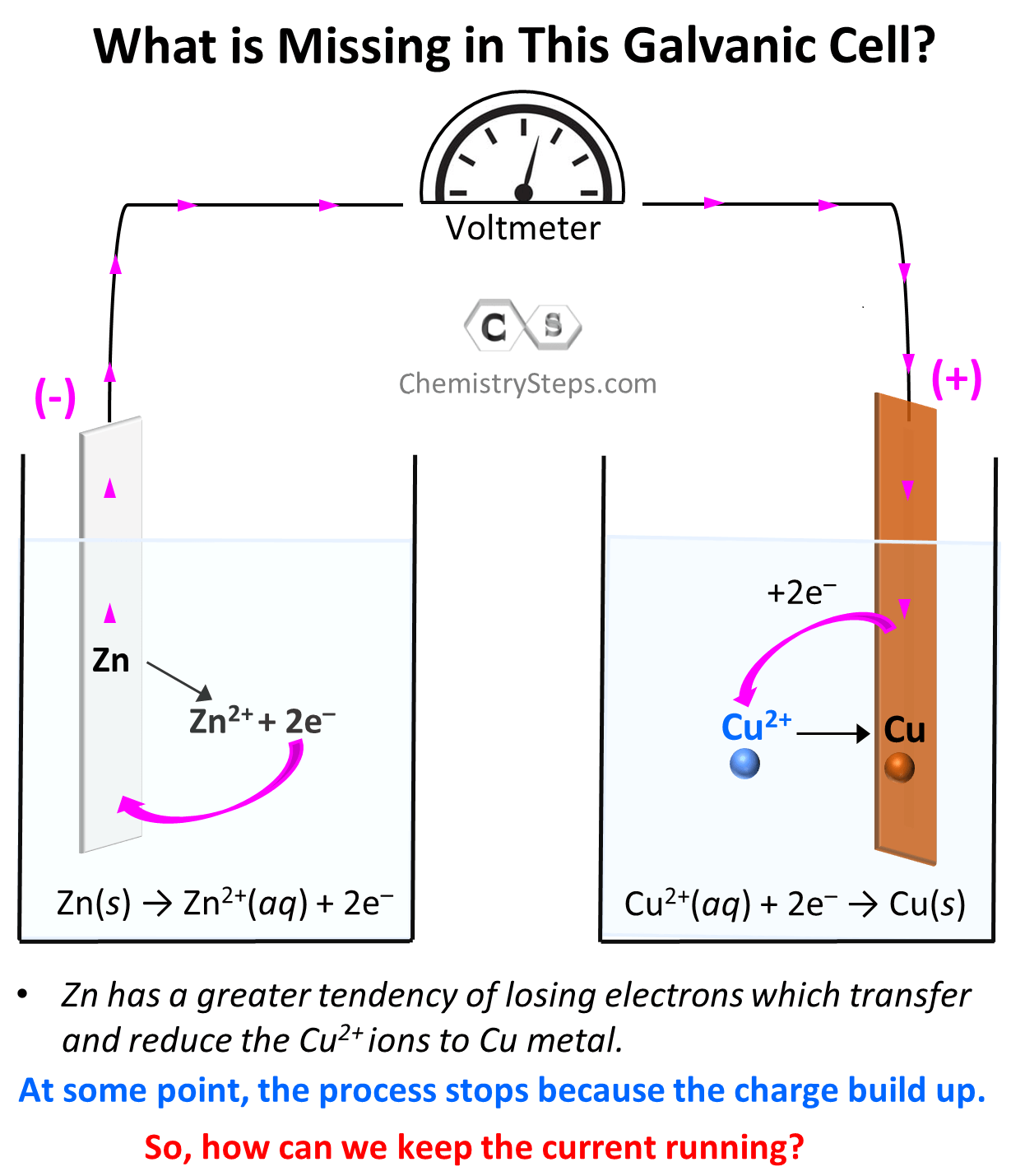
Galvanic Cells Chemistry Steps A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. the cell is comprised of two half cells, each containing the redox conjugate pair (“couple”) of a single reactant. the half cell shown at the left contains the cu (0) cu (ii) couple in the form of a solid copper foil and an aqueous. The design of fuel cells requires extensive knowledge of electrochemistry. galvanic cells, also known as voltaic cells, are electrochemical cells in which spontaneous oxidation reduction reactions produce electrical energy. a battery is a galvanic cell. when two half reactions are separated so that electrical current can flow through an. A galvanic cell is a setup that facilitates redox reactions in a controlled and specific way that generates a current (flow of electrons). voltaic cell, electrochemical cell, and battery are all different names for a galvanic cell. below is a typical galvanic cell that will demonstrate all the important features. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.2.2. the cell is comprised of two half cells, each containing the redox conjugate pair (“couple”) of a single reactant. the half cell shown at the left contains the cu(0) cu(ii) cu (0) cu (ii) couple in the form of a solid copper foil.

Comments are closed.