Full Ketones And Aldehydes Lab Report

Full Ketones And Aldehydes Lab Report Aldehydes and ketones lab report writer matthew estacio reviewer maddie looney editor nora cipkowski. introduction: the purpose of the aldehydes and ketones lab involved identifying an unknown ketone or aldehyde by performing several tests. the tollen’s reagent test was used to identify the presence or absence of an aldehyde (reference 1). Reactions of aldehydes and ketones group 1 lead author: michael pham reviewer: courtney steele editor: taigan white the university of alabama at birmingham department of chemistry ch 238, section q8a to satisfy the lab report under the direction of ta oluwatosin badru 25 february 2023.

Aldehydes And Ketones Lab Report Chegg Carry out the experiment in a well ventilated hood. procedure: rinse the test tubes with distilled water, nitric acid, water, acetone, and water in that order. add 5 ml of 0.1 m silver nitrate to each test tube. pour small amounts of ammonia to the silver nitrate solution and swirl until the solution is a brown color (muddy). Reaction of aldehydes and ketones results and discussion. summary: in this lab, we studied the reaction of aldehydes and ketones and used physical properties, qualitative tests: iodoform test, schiff’s test, and tollen’s test; and nmr spectra to identify our assigned unknown. results: i was assigned unknown 509g. Ketones and aldehydes. the carbonyl group is of central importance in organic chemistry because of its ubiquity. without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). the simplest carbonyl compounds are aldehydes and ketones. Aldehydes contain their carbonyl group at the end of the carbon chain and are susceptible to oxidation. in contrast, ketones contain theirs in the middle of the carbon chain and are resistant to oxidation. jones’s test, tollen’s reagent and iodoform reaction were the three tests used to determine the reactions of aldehydes and ketones.
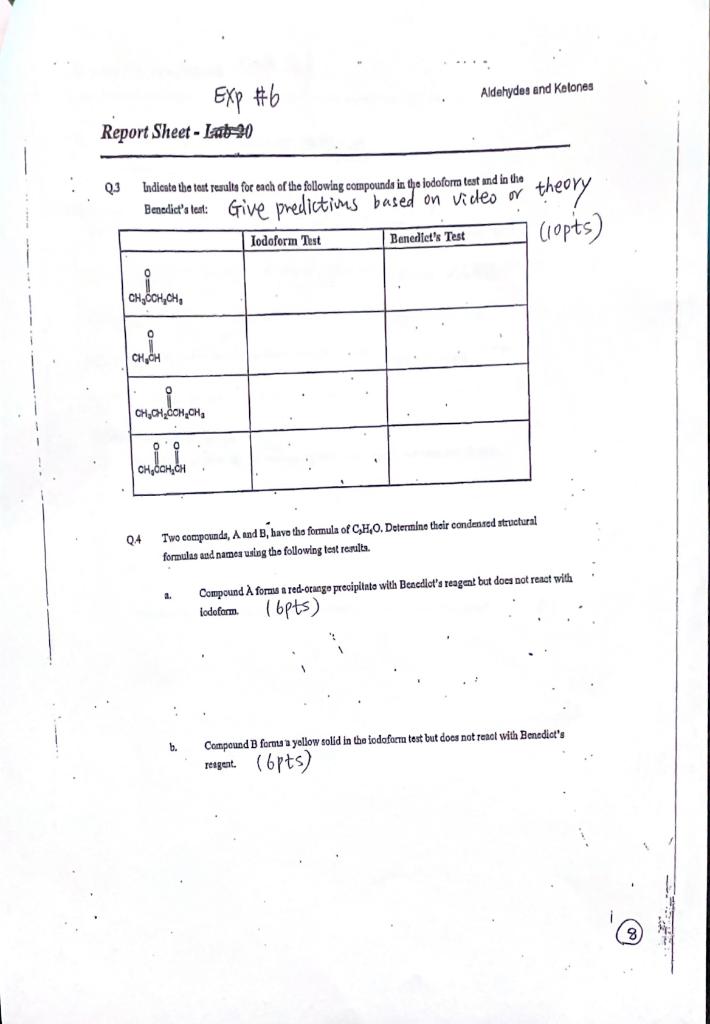
Solved Exp 6 Aldehydes And Ketones Report Sheet Lab 20 Chegg Ketones and aldehydes. the carbonyl group is of central importance in organic chemistry because of its ubiquity. without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). the simplest carbonyl compounds are aldehydes and ketones. Aldehydes contain their carbonyl group at the end of the carbon chain and are susceptible to oxidation. in contrast, ketones contain theirs in the middle of the carbon chain and are resistant to oxidation. jones’s test, tollen’s reagent and iodoform reaction were the three tests used to determine the reactions of aldehydes and ketones. To four small clean, (not rinsed with acetone) but not dry, test tubes add the appropriate test compound (2 4 drops), about 2.5 ml of the base solution provided (naoh) and then add in about 0.75 ml of the iodine solution provided. shake well. record your observations (you may have to cool the test tube in ice). Aldehydes are also oxidized by tollens’ reagent, a substance that contains ag 1. the silver ion is, concomitantly, reduced to metallic silver. silver ion is a weak oxidizing agent; aldehydes are very easily oxidized and are essentially unique in being able to reduce silver ion to silver metal. methyl ketones, but not other ketones, are.

Chem Report 5 Reactions Of Aldehydes And Ketones Docx The Purpose Of To four small clean, (not rinsed with acetone) but not dry, test tubes add the appropriate test compound (2 4 drops), about 2.5 ml of the base solution provided (naoh) and then add in about 0.75 ml of the iodine solution provided. shake well. record your observations (you may have to cool the test tube in ice). Aldehydes are also oxidized by tollens’ reagent, a substance that contains ag 1. the silver ion is, concomitantly, reduced to metallic silver. silver ion is a weak oxidizing agent; aldehydes are very easily oxidized and are essentially unique in being able to reduce silver ion to silver metal. methyl ketones, but not other ketones, are.
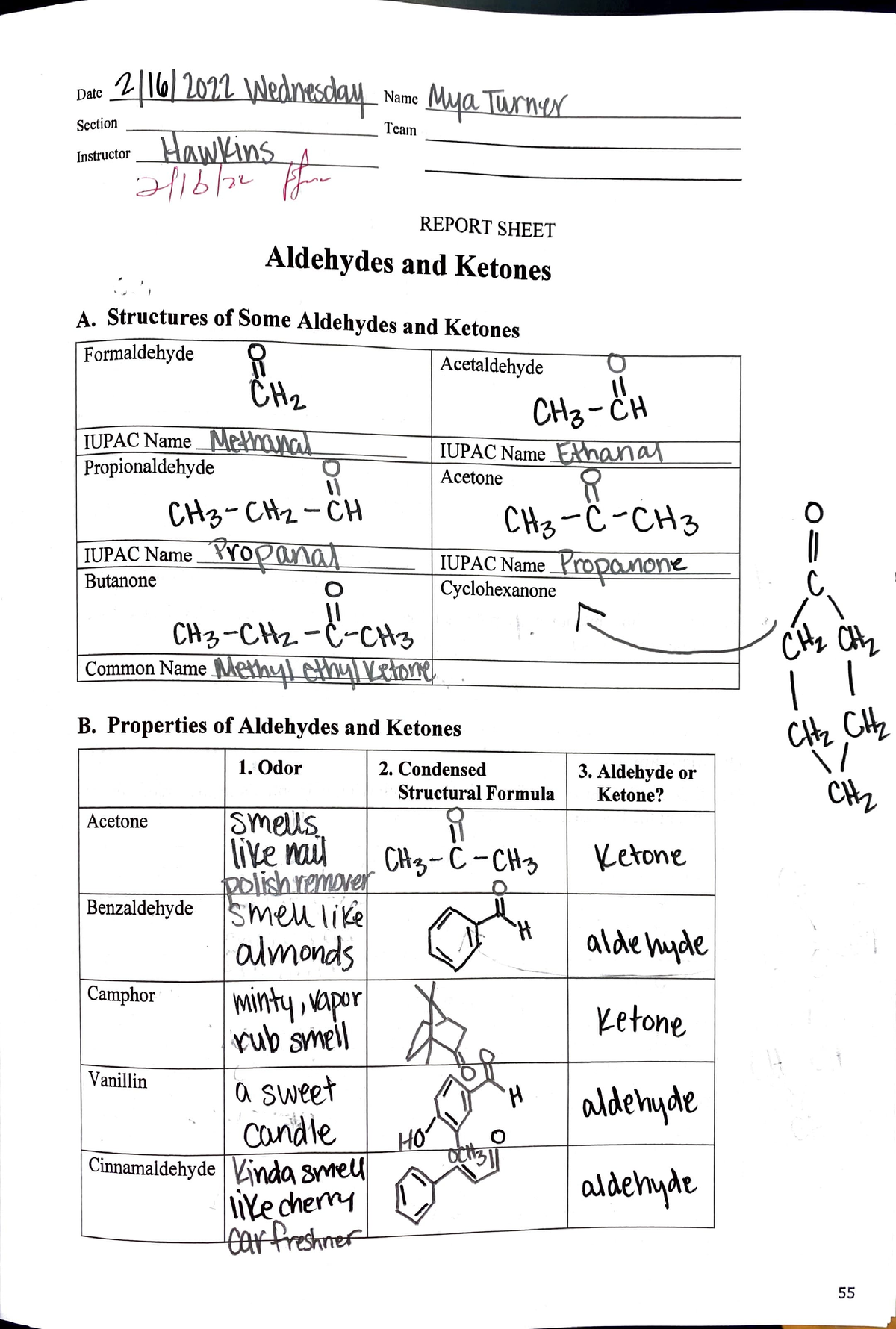
Aldehydes And Ketones Lab Report Date 1 I 1dt L Nw Name

Comments are closed.