Flippin Science Topic 3 9 Soaps And Micelles

Back To Basics What Is Soap And How Does It Work вђ The Macbath In this video i talk about soaps, micelle formation and reverse micelles. Micellessoaps are molecules having two ends with differing properties, one end is hydrophilic, that is, it dissolves in water, while the other end is hydroph.
:max_bytes(150000):strip_icc()/soap-micelle-58ed36193df78cd3fcdf0908.jpg)
How Soap Works A micelle is a spherical structure that forms in water by the aggregation of surfactant molecules, with their hydrophobic (water hating) tails inward and hydrophilic (water loving) heads outward. micelles are like tiny, invisible soap bubbles in solutions. when soap or similar substances dissolve in water, they group together into tiny. The molecule of soap constitutes sodium or potassium salts of long chain carboxylic acids. in the case of soaps, the carbon chain dissolves in oil and the ionic end dissolves in water. thus, the soap molecules form structures called micelles. in micelles, one end is towards the oil droplet and the other end which is the ionic faces outside. Polymeric micelles (pms) are micelles, however, the amphiphilic molecules found in solution are macromolecules. pms have bigger hydrophobic blocks, resulting in lower cmc values compared to ordinary micelles. therefore, pms are more stable in dilute solutions. pms are distinguished from traditional micelles by the following properties. Because soap is a salt, it partially separates into its component ions in water. the active ion of the soap molecule is the rcoo . the two ends of this ion behave in different fashions. the carboxylate end ( coo ) is hydrophilic (water loving), and is said to be the "head" of the ion. the hydrocarbon portion is lipophilic (oil loving) and is.
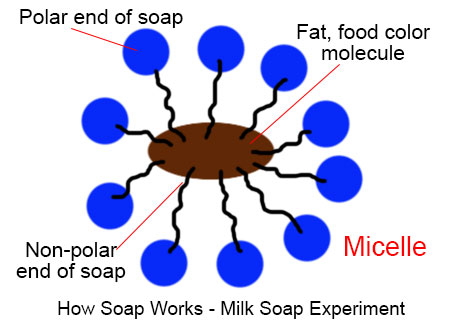
How Soap Works Easy Science Experiment For Kids Polymeric micelles (pms) are micelles, however, the amphiphilic molecules found in solution are macromolecules. pms have bigger hydrophobic blocks, resulting in lower cmc values compared to ordinary micelles. therefore, pms are more stable in dilute solutions. pms are distinguished from traditional micelles by the following properties. Because soap is a salt, it partially separates into its component ions in water. the active ion of the soap molecule is the rcoo . the two ends of this ion behave in different fashions. the carboxylate end ( coo ) is hydrophilic (water loving), and is said to be the "head" of the ion. the hydrocarbon portion is lipophilic (oil loving) and is. The micelle interior is completely nonpolar. spherical bilayers that enclose an aqueous compartment are called vesicles or liposomes. micelles and bilayers, formed from single and double chain amphiphiles, respectively, represent noncovalent aggregates and hence are formed by an entirely physical process. no covalent steps are required. Description of micellar surfactant systems emerges from the phase rule. the number of phases = 3; vapor, aqueous solution and micelle. the number of components = 2; water and surfactant. hence having defined the temperature, the remaining intensive variables are defined; i.e. vapor pressure, mole fraction of surfactant monomer in aqueous.
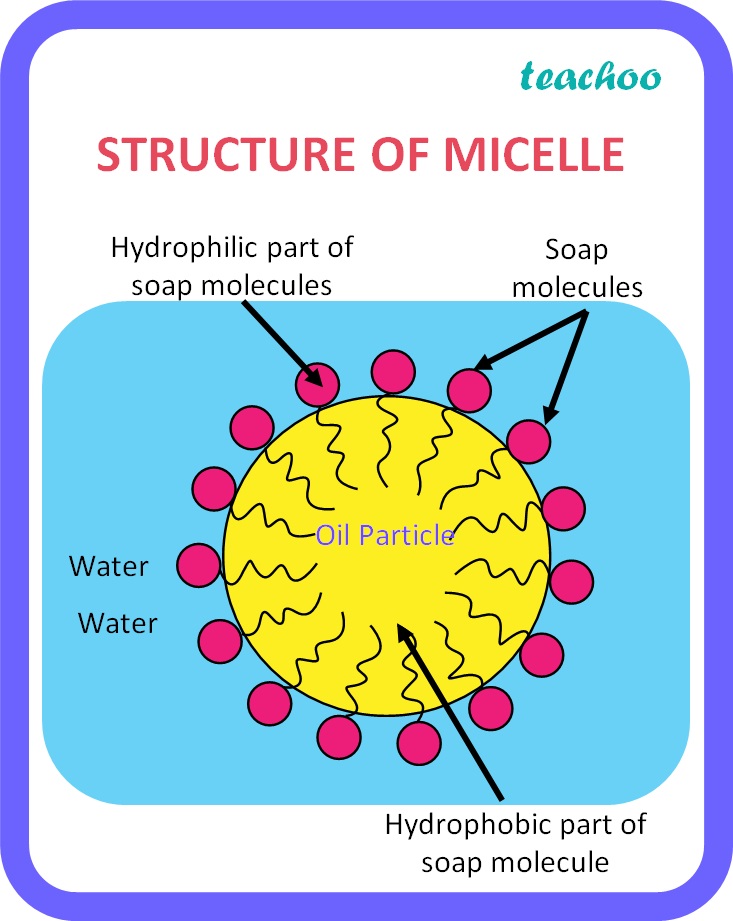
Class 10 Carbon And Its Compounds What Are Micelles Teachoo The micelle interior is completely nonpolar. spherical bilayers that enclose an aqueous compartment are called vesicles or liposomes. micelles and bilayers, formed from single and double chain amphiphiles, respectively, represent noncovalent aggregates and hence are formed by an entirely physical process. no covalent steps are required. Description of micellar surfactant systems emerges from the phase rule. the number of phases = 3; vapor, aqueous solution and micelle. the number of components = 2; water and surfactant. hence having defined the temperature, the remaining intensive variables are defined; i.e. vapor pressure, mole fraction of surfactant monomer in aqueous.

Draw Labelled Diagram Of Soap Micelle Chemistry Shaal Vrogue Co

Comments are closed.