First Order Integrated Rate Law Equation
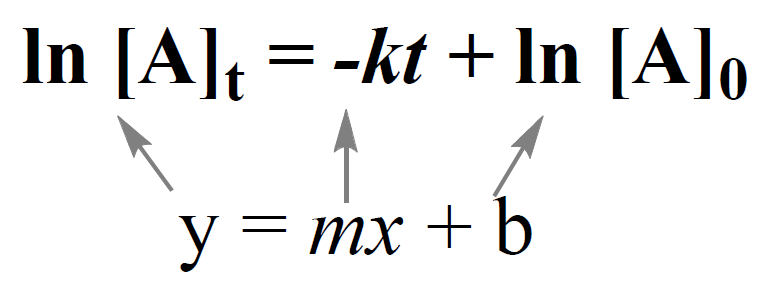
Integrated Rate Law Chemistry Steps The exponential form of the integrated rate law for a first order reaction (equation \(\ref{14.4.6}\)) is [a] = [a] 0 e −kt. a having been given the initial concentration of ethyl chloride ([a] 0) and having the rate constant of k = 1.6 × 10 −6 s −1, we can use the rate law to calculate the concentration of the reactant at a given time t. Example 12.5.3 12.5. 3: the integrated rate law for a second order reaction. the reaction of butadiene gas (c 4 h 6) with itself produces c 8 h 12 gas as follows: 2c4h6(g) c8h12(g) 2 c 4 h 6 (g) c 8 h 12 (g) the reaction is second order with a rate constant equal to 5.76 × 10 −2 l mol min under certain conditions.

Integrated Rate Equation For First Order Reaction First Order Integration of the rate law for a simple first order reaction (rate = k[a]) results in an equation describing how the reactant concentration varies with time: \[[a] t=[a] 0 e^{ k t} \nonumber \] where [a]t is the concentration of a at any time t, [a] 0 is the initial concentration of a, and k is the first order rate constant. For purposes of discussion, we will focus on the resulting integrated rate laws for first , second , and zero order reactions. first order reactions. integration of the rate law for a simple first order reaction (rate = k[a]) results in an equation describing how the reactant concentration varies with time:. In this video, we'll use the first order integrated rate law to calculate the concentration of a reactant after a given amount of time. we'll also calculate the amount of time it takes for the concentration to decrease to a certain value. finally, we'll use the first order half life equation to calculate the half life of the reaction. The integrated rate law for a zero order reaction also has the form of the equation of a straight line: [a]t = − kt [a]0 y = mx b. as shown in figure 18.4.6, a plot of [a] versus t for a zero order reaction is a straight line with a slope of − k and a y intercept of [a] 0. figure 18.4.6.
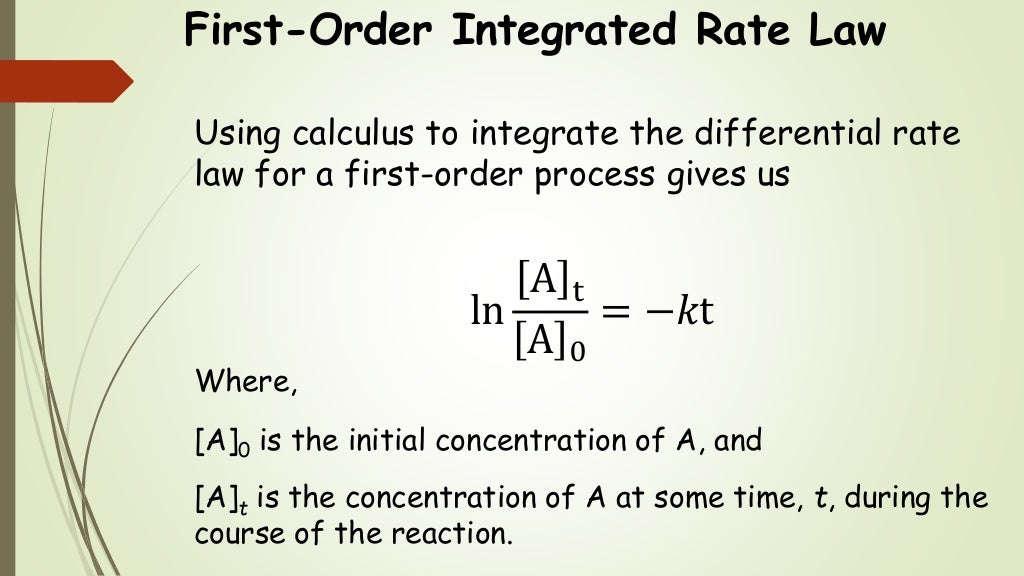
Chem 2 Chemical Kinetics Iv The First Order Integrated Rate Law In this video, we'll use the first order integrated rate law to calculate the concentration of a reactant after a given amount of time. we'll also calculate the amount of time it takes for the concentration to decrease to a certain value. finally, we'll use the first order half life equation to calculate the half life of the reaction. The integrated rate law for a zero order reaction also has the form of the equation of a straight line: [a]t = − kt [a]0 y = mx b. as shown in figure 18.4.6, a plot of [a] versus t for a zero order reaction is a straight line with a slope of − k and a y intercept of [a] 0. figure 18.4.6. Integration of the rate law for a simple first order reaction $(rate=k[a])$ results in an equation describing how the reactant concentration varies with time: $$ ln(\frac{[a] 0}{ [a] t })=kt \label{eq1}\tag{1}$$ where k is the rate constant, the initial concentration is [a] 0 and [a] t is the concentration present after any given time t. this. Using the first order integrated rate law and half life.

First Order Integrated Rate Law Equation Gallery Dedemax Integration of the rate law for a simple first order reaction $(rate=k[a])$ results in an equation describing how the reactant concentration varies with time: $$ ln(\frac{[a] 0}{ [a] t })=kt \label{eq1}\tag{1}$$ where k is the rate constant, the initial concentration is [a] 0 and [a] t is the concentration present after any given time t. this. Using the first order integrated rate law and half life.

Comments are closed.