First Law Of Thermodynamics Basic Introduction Internal Energy Heat

First Law Of Thermodynamics Basic Introduction Internal Energy Heat This chemistry video tutorial provides a basic introduction into the first law of thermodynamics. it shows the relationship between internal energy, heat, a. The first law of thermodynamics applies the conservation of energy principle to systems where heat transfer and doing work are the methods of transferring energy into and out of the system. the first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system.
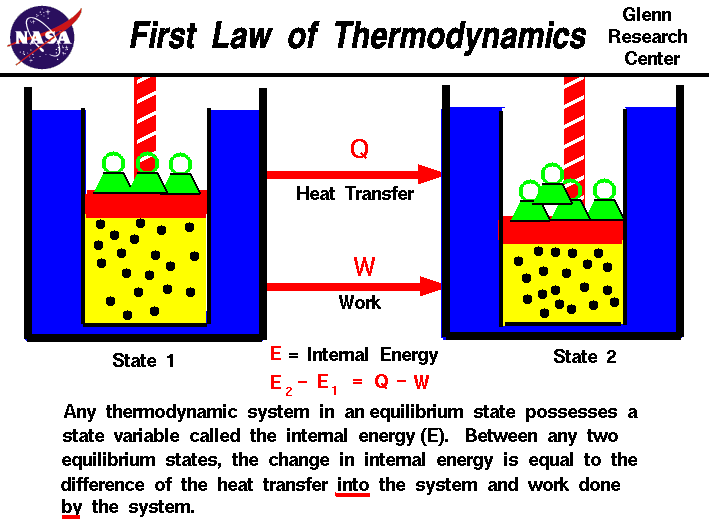
First Law Of Thermodynamics The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. in equation form, the first law of thermodynamics is. Δu = q − w. (15.1.1) (15.1.1) Δ u = q − w. here Δu Δ u is the change in internal energy u u of the system. The first law of thermodynamics is: the increase of the internal energy of a system is equal to the sum of the heat added to the system plus the work done on the system. in symbols: du = dq dw (7.1.1) (7.1.1) d u = d q d w. you may regard this, according to taste, as any of the following. The first law of thermodynamics states that the change in internal energy of a closed system equals the net heat transfer into the system minus the net work done by the system. in equation form, the first law of thermodynamics is. Δu = q − w. Δ u = q − w. 12.6. (a) because the system is an ideal gas, the internal energy only changes when the temperature changes. (b) the heat added to the system is therefore purely used to do work that has been calculated in work, heat, and internal energy. (c) lastly, the first law of thermodynamics can be used to calculate the heat added to the gas. solution.

Comments are closed.