Find The Vapour Pressure Clausius Clapeyron Equation

Clausius Clapeyron Equation A simple relationship can be found by integrating equation 1 1 between two pressure temperature endpoints: ln(p1 p2) = Δhvap r (1 t2 − 1 t1) (2) (2) ln (p 1 p 2) = Δ h v a p r (1 t 2 − 1 t 1) where p1 p 1 and p2 p 2 are the vapor pressures at two temperatures t1 t 1 and t2 t 2. equation 2 2 is known as the clausius clapeyron equation and. We can use the omnicalculator tool vapor pressure calculator or the clausius clapeyron equation as follows: define a boiling temperature and pressure you know. let's say 100 °c, at 101.3 kpa of atmospheric pressure. find out the new pressure at which water will boil. hint, air pressure at 3500 m.a.s.l., at 20 °c is 65 kpa.

Chemistry 201 Using The Clausius Clapeyron Equation To Solve For Vapor Equation 23.4.1 is known as the clausius clapeyron equation. we can further work our the integration and find the how the equilibrium vapor pressure changes with temperature: ln(p2 p1) = − Δhvap molar r [1 t2 − 1 t1] thus if we know the molar enthalpy of vaporization we can predict the vapor lines in the diagram. The clausius–clapeyron equation [7]: 509 applies to vaporization of liquids where vapor follows ideal gas law using the specific gas constant and liquid volume is neglected as being much smaller than vapor volume v. it is often used to calculate vapor pressure of a liquid. The clausius clapeyron equation states that the logarithm of the vapor pressure of a substance is directly proportional to its reciprocal absolute temperature. according to it, the natural logarithm of the liquid’s vapor pressure (p) is determined by the molar enthalpy of vaporization of the liquid (Δh vap ), the ideal gas constant (r), and the temperature (t) of the system. Clausius clapeyron equation example problem. for example, we can use the clausius clapeyron equation for predicting the vapor pressure of a solution. calculate the vapor pressure of 1 propanol at 52.8 °c if the vapor pressure is 10.0 torr at 14.7 °c and its heat of vaporization is 47.2 kj mol.
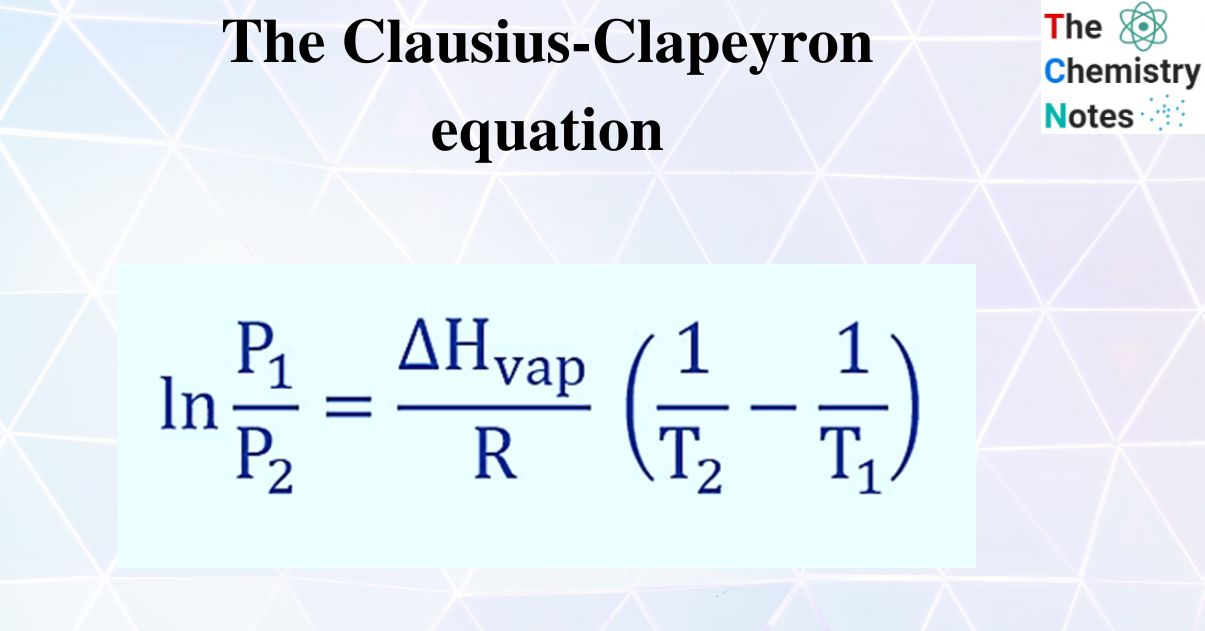
The Clausius Clapeyron Equation Derivation With Applications The clausius clapeyron equation states that the logarithm of the vapor pressure of a substance is directly proportional to its reciprocal absolute temperature. according to it, the natural logarithm of the liquid’s vapor pressure (p) is determined by the molar enthalpy of vaporization of the liquid (Δh vap ), the ideal gas constant (r), and the temperature (t) of the system. Clausius clapeyron equation example problem. for example, we can use the clausius clapeyron equation for predicting the vapor pressure of a solution. calculate the vapor pressure of 1 propanol at 52.8 °c if the vapor pressure is 10.0 torr at 14.7 °c and its heat of vaporization is 47.2 kj mol. 1) let us use the clausius clapeyron equation: ln (x 1.00) = (30800 8.31447) (1 353.25 minus 1 298.65) comment: i used the form of the equation shown in this image: i assigned the unknown value to be associated with p 2. that puts x in the numerator and a 1.00 in the denominator, making my calculation a bit easier. 2) let us calculate:. Next, calculate the difference in temperature (Δt = t2 – t1) and the difference in vapor pressure (Δp = p2 – p1). finally, use the clausius clapeyron equation: ln (p2 p1) = Δhvap r * (1 t2 – 1 t1), where Δhvap is the enthalpy of vaporization and r is the gas constant. after inserting the values and calculating the result, check your.
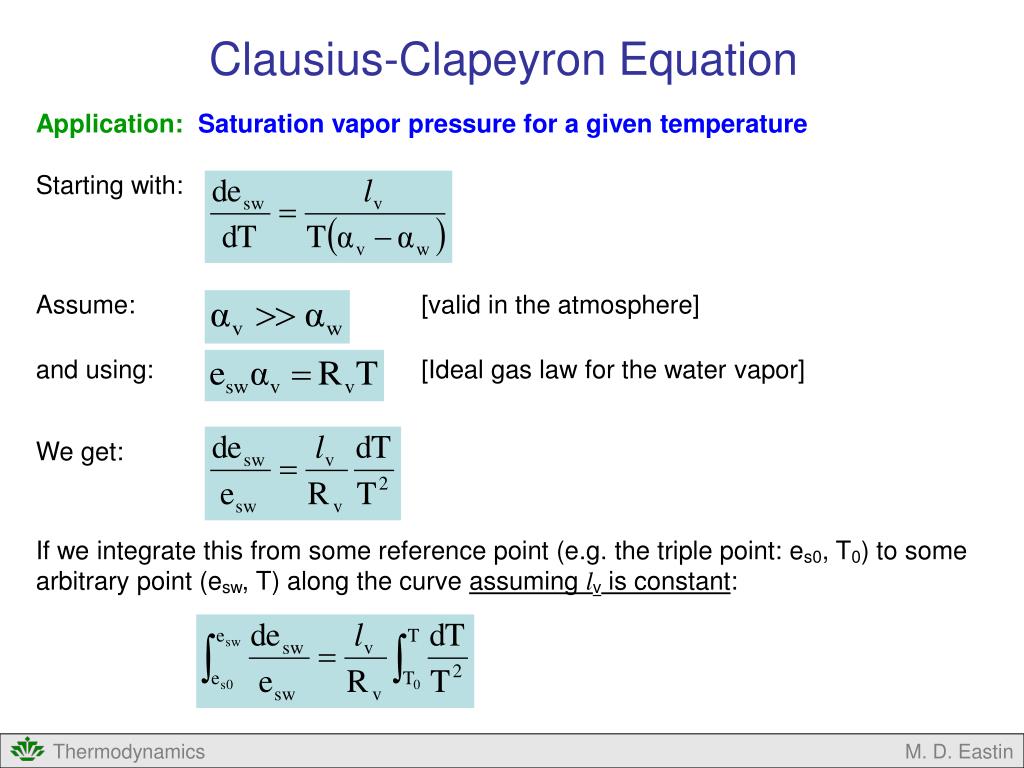
Ppt Clausius Clapeyron Equation Powerpoint Presentation Free 1) let us use the clausius clapeyron equation: ln (x 1.00) = (30800 8.31447) (1 353.25 minus 1 298.65) comment: i used the form of the equation shown in this image: i assigned the unknown value to be associated with p 2. that puts x in the numerator and a 1.00 in the denominator, making my calculation a bit easier. 2) let us calculate:. Next, calculate the difference in temperature (Δt = t2 – t1) and the difference in vapor pressure (Δp = p2 – p1). finally, use the clausius clapeyron equation: ln (p2 p1) = Δhvap r * (1 t2 – 1 t1), where Δhvap is the enthalpy of vaporization and r is the gas constant. after inserting the values and calculating the result, check your.

Comments are closed.