Fda Approval To Initiate Nanoknife Direct Clinical Study
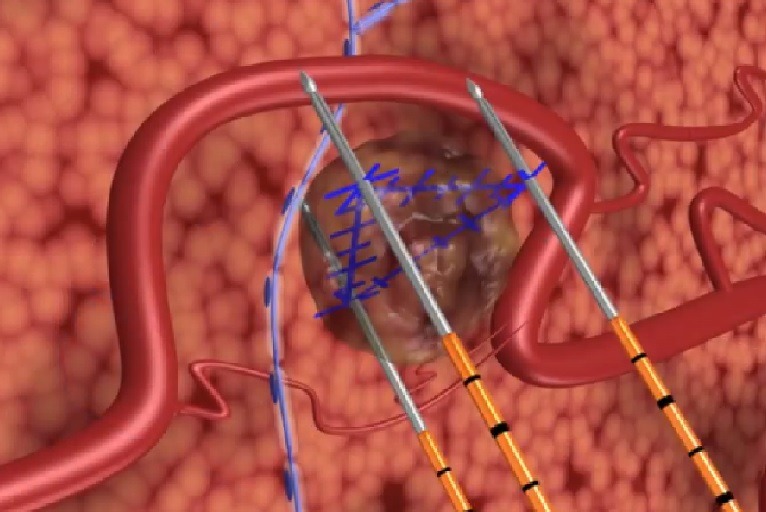
Fda Approval To Initiate Nanoknife Direct Clinical Study The nanoknife system received 510(k) clearance from the fda for the surgical ablation of soft tissue in 2008. unlike other ablative technologies, the nanoknife system utilises low energy, direct current electrical pulses to permanently open pores in target cell membranes and does not rely on thermal effects, the press release explains. The direct study supports a proposed expanded indication for the nanoknife system in the treatment of stage iii pancreatic cancer. in january 2018 , the fda granted the company’s nanoknife system a breakthrough device designation under the 21st century cures act.

Nanoknife Virtual Booth Angiodynamics Nanoknife Ire System Virtual Booth Angiodynamics’ direct study will feature a comprehensive data collection strategy that will provide meaningful clinical information to healthcare professionals, support a regulatory indication for the treatment of stage iii pancreatic cancer, and facilitate reimbursement for hospitals and treating physicians. In january 2018, the fda granted the company’s nanoknife system a breakthrough device designation under the 21st century cures act. “the direct study design demonstrates our commitment to provide an option that addresses a pressing unmet need for patients with stage iii pancreatic cancer,” said jim clemmer, president and chief executive. In the open label, single group assignment preserve study, all participants receive nanoknife ablation of the prostate tissue. to enroll in the trial patients, had to be ≥50 years old with histologically confirmed, organ confined prostate cancer (≤t2c). patients had to have a gleason score of 3 4 or 4 3 and a psa level ≤15 ng ml or a psa. The latest lde approval will enable angiodynamics to initiate its direct clinical study, which will evaluate the nanoknife system in around 250 patients with stage iii pancreatic cancer. the study will involve a series of randomised controlled trials (rct) in up to 15 clinical sites and real world evidence, next generation registry (rwe) at up.

Comments are closed.