Factors Affecting Reaction Rates

Factors Affecting Reaction Rate вђ Overview Examples Expii The rates at which reactants are consumed and products are formed during chemical reactions vary greatly. we can identify five factors that affect the rates of chemical reactions: the chemical nature of the reacting substances, the state of subdivision (one large lump versus many small particles) of the reactants, the temperature of the reactants, the concentration of the reactants, and the. The rate of reaction was discussed in terms of three factors: collision frequency, the collision energy, and the geometric orientation. remember that the collision frequency is the number of collisions per second. the collision frequency is dependent, among other factors, on the temperature of the reaction.

Ppt Rate Of Reaction Powerpoint Presentation Free Download Id 2483456 Use the phet reactions & rates interactive to explore how temperature, concentration, and the nature of the reactants affect reaction rates. this page titled 12.2: factors affecting reaction rates is shared under a cc by 4.0 license and was authored, remixed, and or curated by openstax via source content that was edited to the style and. Summary of factors that affect reaction rate. factor. affect on reaction rate. temperature. increasing temperature increases reaction rate (up to a point) pressure. increasing pressure of gases increases reaction rate. concentration. increasing the amount of reactants in a solution increases reaction rate. The rates at which reactants are consumed and products are formed during chemical reactions vary greatly. five factors typically affecting the rates of chemical reactions will be explored in this section: the chemical nature of the reacting substances, the state of subdivision (one large lump versus many small particles) of the reactants, the temperature of the reactants, the concentration of. Learn how collision theory, activation energy, and four main factors (reactant concentration, physical state, temperature, and catalyst) influence the rate of chemical reactions. see examples, visualizations, and diagrams to illustrate the concepts.
Ppt Factors Affecting Reaction Rates Powerpoint Presentation Free The rates at which reactants are consumed and products are formed during chemical reactions vary greatly. five factors typically affecting the rates of chemical reactions will be explored in this section: the chemical nature of the reacting substances, the state of subdivision (one large lump versus many small particles) of the reactants, the temperature of the reactants, the concentration of. Learn how collision theory, activation energy, and four main factors (reactant concentration, physical state, temperature, and catalyst) influence the rate of chemical reactions. see examples, visualizations, and diagrams to illustrate the concepts. Learn how the chemical nature, physical state, temperature, concentration, and catalysis of reactants influence the rates of chemical reactions. see examples, videos, and interactive simulations to explore the effects of these factors on reaction rates. Learn how concentration, temperature, medium, catalysts, pressure, and mixing influence the rate of chemical reactions. see a chart summarizing the main factors and their effects on reaction rate.
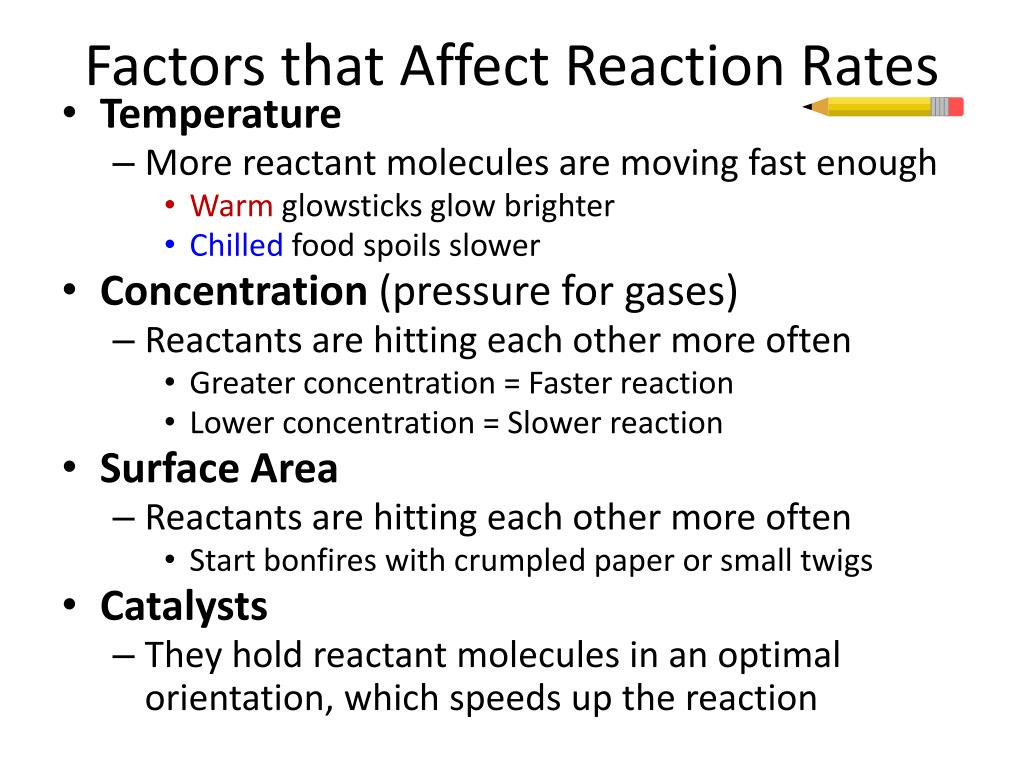
Ppt Reaction Rates Powerpoint Presentation Free Download Id 6516140 Learn how the chemical nature, physical state, temperature, concentration, and catalysis of reactants influence the rates of chemical reactions. see examples, videos, and interactive simulations to explore the effects of these factors on reaction rates. Learn how concentration, temperature, medium, catalysts, pressure, and mixing influence the rate of chemical reactions. see a chart summarizing the main factors and their effects on reaction rate.

Comments are closed.