F2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo

F2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Fluorine, with the chemical formula f2, is a pale yellow colored diatomic gas, which has a pungent odor. f2 has a molecular weight of 37.997 g mol. its boiling point is −188 °c, and its melting point is −219.67 °c. it is toxic in nature; it can cause chemical burns on the skin and can be lethal if inhaled. it is highly reactive, is. The lewis structure is a representation of a molecule’s valence electrons using dots and lines to indicate bonds. for the f2 molecule, there are two fluorine atoms bonded together through a single covalent bond. each fluorine atom has 7 valence electrons, and they share one electron pair to form the bond.

F2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo 4.2: molecular structure and polarity. vsepr theory predicts the three dimensional arrangement of atoms in a molecule. it states that valence electrons will assume an electron pair geometry that minimizes repulsions between areas of high electron density (bonds and or lone pairs). molecular structure, which refers only to the placement of atoms. Xef2 lewis structure, molecular geometry, hybridization, and mo diagram. xef2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. this is an active solvent and is found to be soluble in different fluorides like hf and bromine pentafluoride. if we look at the process of synthesis of xenon difluoride, here’s. Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals. mix at least 2 nonequivalent atomic orbitals (e.g. s and p). hybrid orbitals have very different shape from original atomic orbitals. number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. To summarize this blog, we can say that: in the lewis structure of of2, both fluorine atoms share a single bond with the oxygen. the central oxygen atom has two lone pairs of electrons, and the bond angle of f o f is 109° 27′. it has a linear molecular geometry and sp3 hybridization. of2 has a bent shape and a tetrahedral electron geometry.

Lewis Structure Shape Hybridization And Polarity Prac Vrogue Co Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals. mix at least 2 nonequivalent atomic orbitals (e.g. s and p). hybrid orbitals have very different shape from original atomic orbitals. number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. To summarize this blog, we can say that: in the lewis structure of of2, both fluorine atoms share a single bond with the oxygen. the central oxygen atom has two lone pairs of electrons, and the bond angle of f o f is 109° 27′. it has a linear molecular geometry and sp3 hybridization. of2 has a bent shape and a tetrahedral electron geometry. Step 1: determining the total number of valence electrons in the molecule. the valence electron for carbon (1s22s22p2) and hydrogen (1s1) is 4 and 1, respectively. in ethane, we have two carbon atoms and 6 hydrogen atoms and hence, the total number of valence electron are (2 x 4) (1 x 6) = 14. step 2: drawing the lewis structure:. Contributors. 10.4: geometry and molecular polarity is shared under a license and was authored, remixed, and or curated by libretexts. vsepr theory predicts the three dimensional arrangement of atoms in a molecule. it states that valence electrons will assume an electron pair geometry that minimizes repulsions between areas of high ….
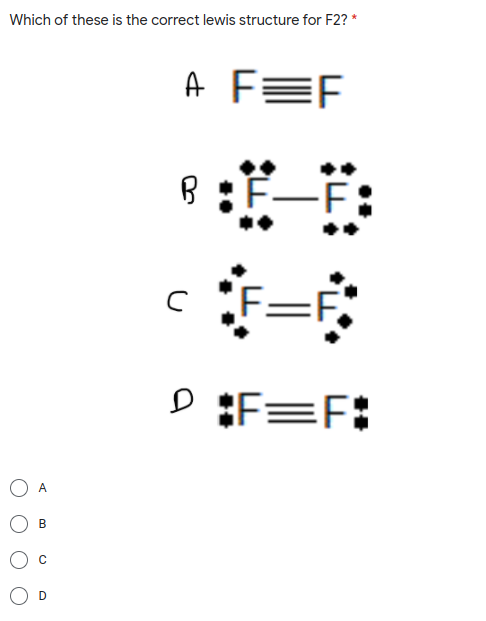
F2 Lewis Dot Structure Step 1: determining the total number of valence electrons in the molecule. the valence electron for carbon (1s22s22p2) and hydrogen (1s1) is 4 and 1, respectively. in ethane, we have two carbon atoms and 6 hydrogen atoms and hence, the total number of valence electron are (2 x 4) (1 x 6) = 14. step 2: drawing the lewis structure:. Contributors. 10.4: geometry and molecular polarity is shared under a license and was authored, remixed, and or curated by libretexts. vsepr theory predicts the three dimensional arrangement of atoms in a molecule. it states that valence electrons will assume an electron pair geometry that minimizes repulsions between areas of high ….

Comments are closed.