Explain The Difference Between The Processes Of Melting And Freezing

Explain The Difference Between The Processes Of Melting And Freezing Video 13.3.1 13.3. 1: solid co 2, also known as dry ice, goes directly from a solid phase to a gaseous phase in a process called sublimation. the reverse process of sublimation is called deposition, a process in which a gas goes directly to a solid, bypassing the liquid phase. Differences between melting and freezing. gradual change of state from solid to liquid by absorbing heat from surroundings is called melting. gradual change of state from liquid to solid by leaving heat to surroundings is termed as freezing. the temperature at which solid transforms to liquid is called melting point of the concerned substance.
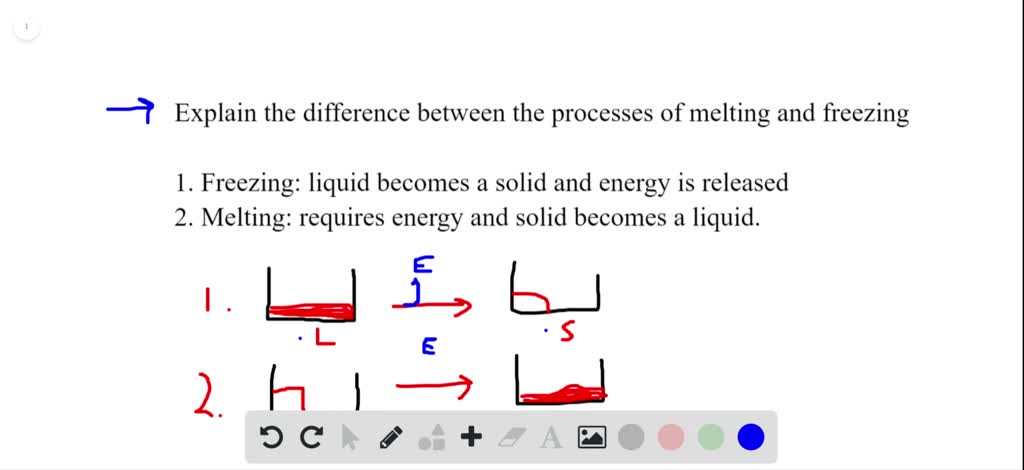
Solved Explain The Difference Between The Processes Of Melting And In short, melting and freezing are opposite processes that involve a change in the state of matter, with melting being the transition from solid to liquid as energy is absorbed, and freezing being the transition from liquid to solid as energy is released. they both occur at specific temperature points and can happen simultaneously at equilibrium. Intermolecular forces have a strong influence on melting point. 12.5: melting, freezing, and sublimation is shared under a ck 12 license and was authored, remixed, and or curated by marisa alviar agnew & henry agnew. phase changes can occur between any two phases of matter. The melting process. melting is the process of changing a solid substance into a liquid. during the melting process, heat is added to a substance, increasing the amount of energy in the particles. This page titled 9.3.1: melting, freezing, and sublimation is shared under a mixed license and was authored, remixed, and or curated by anonymous. phase changes can occur between any two phases of matter. all phase changes occur with a simultaneous change in energy. all phase changes are isothermal.

Give Their Differences Between Melting And Freezing Using A Venn The melting process. melting is the process of changing a solid substance into a liquid. during the melting process, heat is added to a substance, increasing the amount of energy in the particles. This page titled 9.3.1: melting, freezing, and sublimation is shared under a mixed license and was authored, remixed, and or curated by anonymous. phase changes can occur between any two phases of matter. all phase changes occur with a simultaneous change in energy. all phase changes are isothermal. Cooling. if water (liquid) is cooled, it changes to ice (solid). this change is called freezing. water freezes at 0°c. times tables 1 12! what are freezing and melting? find out about the. Freezing and melting. freezing is the change that occurs when a liquid changes into a solid as the temperature decreases. melting is the opposite change, from a solid to a liquid as the temperature increases. these are both examples of changes in the states of matter of substances. substances freeze at exactly the same temperature as they melt.

Comments are closed.