Examples Of Nonmetals

Metals Non Metals And Metalloids Meaning Difference Teachoo Learn what non metals are, how they differ from metals, and what are their physical and chemical properties. find out the complete list of non metals with examples, such as hydrogen, carbon, nitrogen, oxygen, chlorine, etc. The nonmetal element group consists of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur and selenium. hydrogen acts as a nonmetal at normal temperatures and pressure and is generally accepted to be part of the nonmetal group. the halogens are nonmetals in group 7 of the periodic table. atoms of these elements have the 1 oxidation state.
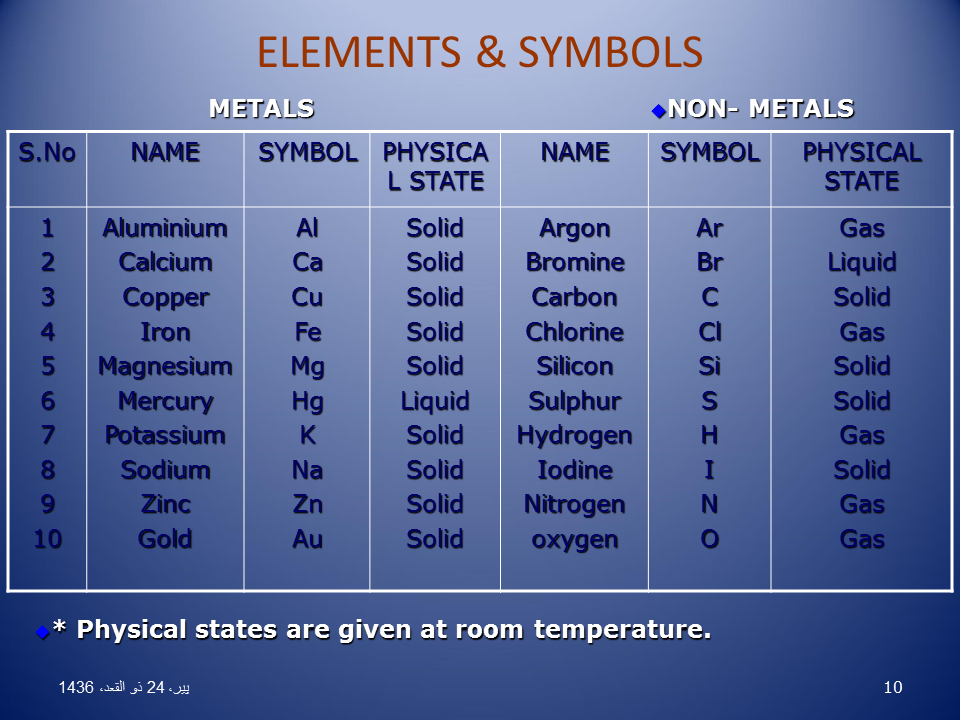
List Of Non Metals With Symbols And Their Uses In Periodic Table Metallic nonmetals . nonmetal elements are classified based on their properties under ordinary conditions. metallic character isn't an all or nothing property. carbon, for example, has allotropes that behave more like metals than nonmetals. sometimes this element is considered to be a metalloid rather than a nonmetal. Nonmetal, in physics, a substance having a finite activation energy (band gap) for electron conduction. this means that nonmetals display low to moderate bulk electrical conductivities, which increase with increasing temperature, and are subject to dielectric breakdown at high voltages and temperatures. Nonmetals are elements that do not display the properties of metals, such as luster, malleability, conductivity, and high melting points. learn about the nonmetal elements, their locations on the periodic table, and their chemical and physical characteristics. For example, the chemically very active nonmetals fluorine, chlorine, bromine, and iodine have an average electronegativity of 3.19—a figure [i] higher than that of any metallic element. the chemical distinctions between metals and nonmetals is connected to the attractive force between the positive nuclear charge of an individual atom and its.
.PNG)
Element Classes Presentation Chemistry Nonmetals are elements that do not display the properties of metals, such as luster, malleability, conductivity, and high melting points. learn about the nonmetal elements, their locations on the periodic table, and their chemical and physical characteristics. For example, the chemically very active nonmetals fluorine, chlorine, bromine, and iodine have an average electronegativity of 3.19—a figure [i] higher than that of any metallic element. the chemical distinctions between metals and nonmetals is connected to the attractive force between the positive nuclear charge of an individual atom and its. A nonmetal is an element that is generally a poor conductor of heat and electricity. most properties of nonmetals are the opposite of metals. there is a wider variation in properties among the nonmetals than among the metals. nonmetals exist in all three states of matter. the majority are gases, such as nitrogen and oxygen. In normal chemical processes, nonmetals do not form monatomic positive ions (cations) because their ionization energies are too high. all monatomic nonmetal ions are anions; examples include the chloride ion, cl −, the nitride ion, n 3−, and the selenide ion, se 2 −. the common oxidation states that the nonmetals exhibit in their ionic.

Comments are closed.