Effects Of Temperature And Pressure On Solubility

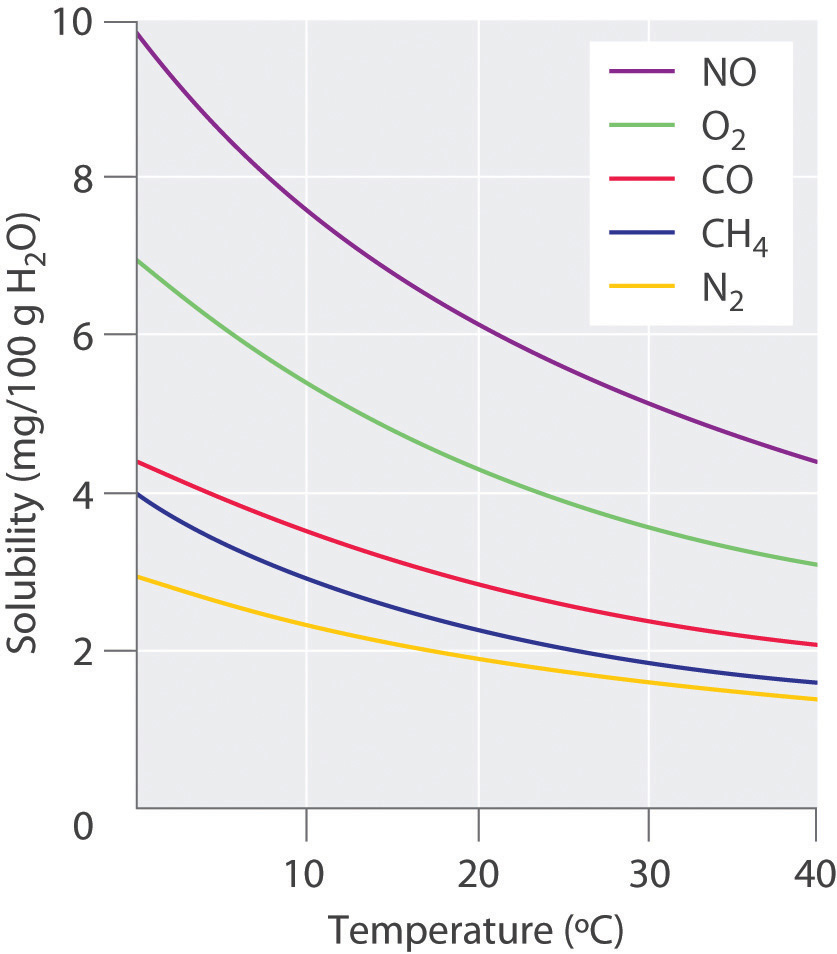
The Effect Of Temperature And Pressure On Solubility Mr C Youtube According to the temperature curves in figure 13.4.1, both compounds dissolve in water at 80°c, and all 50 g of kbr remains in solution at 0°c. only about 36 g of ch 3co 2na are soluble in 100 g of water at 0°c, however, so approximately 114 g (150 g − 36 g) of ch 3co 2na crystallizes out on cooling. Figure 13.3.2 shows how the solubility of a gas in water goes down as the temperature is raised. this decrease in the solubility of oxygen as temperature goes up is one of the reasons cold water fish like trout can not live in warm water. figure 13.4.2 13.4. 2: solubility of several gasses in water.
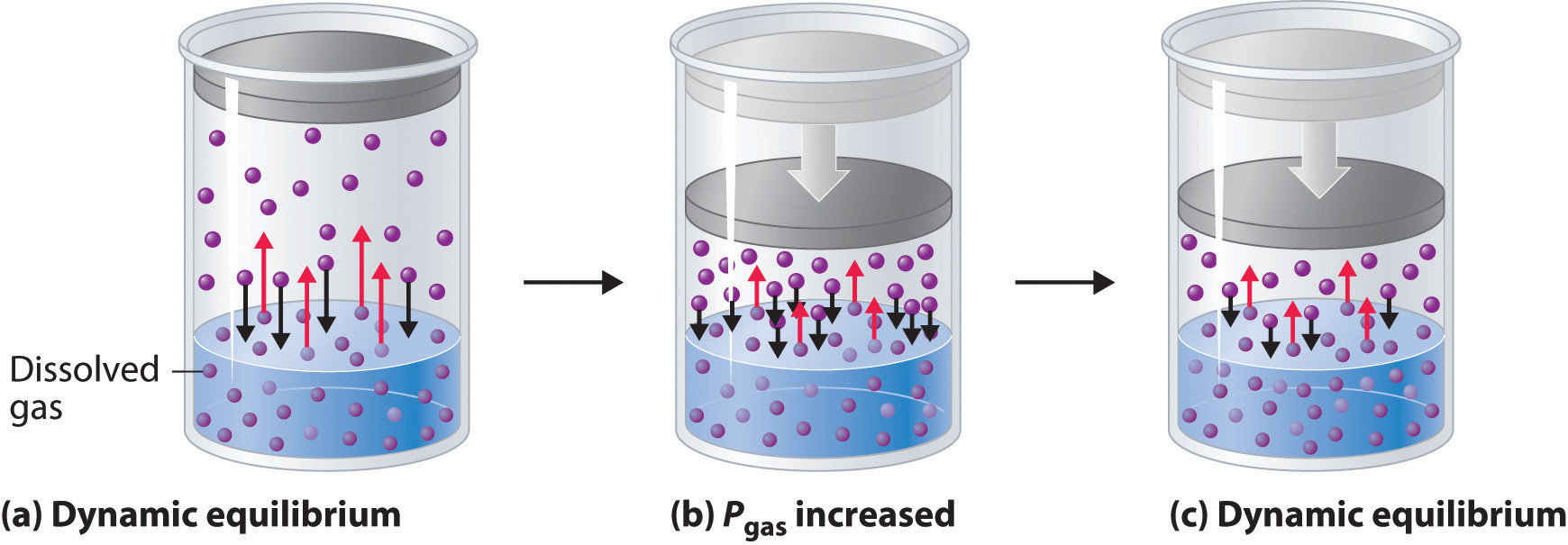
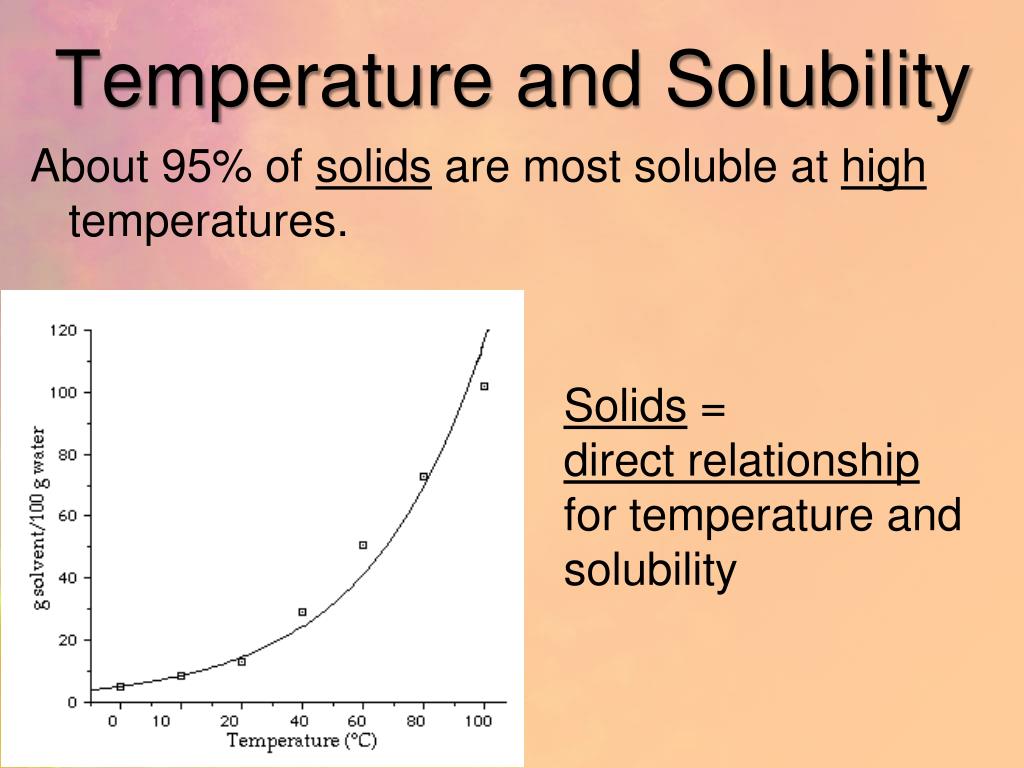
13 4 Pressure And Temperature Effects On Solubility Chemistry Libretexts Effect of temperature on the solubility of gases. the solubility of gases in liquids decreases with increasing temperature, as shown in figure 13.10 "solubilities of several common gases in water as a function of temperature at partial pressure of 1 atm". attractive intermolecular interactions in the gas phase are essentially zero for most. The solubility of solutes is dependent on temperature. when a solid dissolves in a liquid, a change in the physical state of the solid analogous to melting takes place. heat is required to break the bonds holding the molecules in the solid together. at the same time, heat is given off during the formation of new solute solvent bonds. The dependence of solubility on temperature for a number of inorganic solids in water is shown by the solubility curves in figure 9. reviewing these data indicate a general trend of increasing solubility with temperature, although there are exceptions, as illustrated by the ionic compound cerium sulfate. According to henry’s law, for an ideal solution the solubility, cg, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly proportional to the pressure, pg, of the undissolved gas above the solution (101.3 kpa in this case). because both cg and pg are known, this relation can be rearranged and used to solve for k.
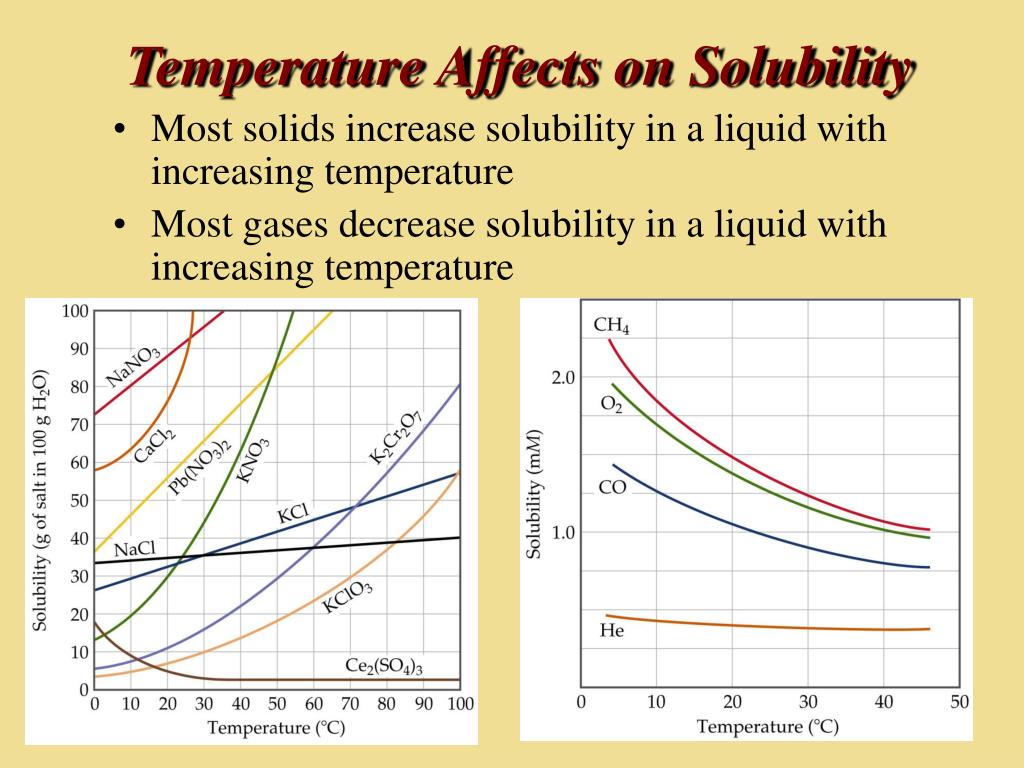
Ppt Solubility Powerpoint Presentation Free Download Id 37453 The dependence of solubility on temperature for a number of inorganic solids in water is shown by the solubility curves in figure 9. reviewing these data indicate a general trend of increasing solubility with temperature, although there are exceptions, as illustrated by the ionic compound cerium sulfate. According to henry’s law, for an ideal solution the solubility, cg, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly proportional to the pressure, pg, of the undissolved gas above the solution (101.3 kpa in this case). because both cg and pg are known, this relation can be rearranged and used to solve for k. The effect of temperature on solubility can be explained on the basis of le chatelier's principle. le chatelier's principle states that if a stress (for example, heat, pressure, concentration of one reactant) is applied to an equilibrium, the system will adjust, if possible, to minimize the effect of the stress. According to henry’s law, for an ideal solution the solubility, cg, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly proportional to the pressure, pg, of the undissolved gas above the solution (101.3 kpa, or 760 torr, in this case). because we know both cg and pg, we can rearrange this expression to solve for k.
Effects Of Temperature And Pressure On Solubility The effect of temperature on solubility can be explained on the basis of le chatelier's principle. le chatelier's principle states that if a stress (for example, heat, pressure, concentration of one reactant) is applied to an equilibrium, the system will adjust, if possible, to minimize the effect of the stress. According to henry’s law, for an ideal solution the solubility, cg, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly proportional to the pressure, pg, of the undissolved gas above the solution (101.3 kpa, or 760 torr, in this case). because we know both cg and pg, we can rearrange this expression to solve for k.
Ppt Factors Affecting Solubility Powerpoint Presentation Free

Comments are closed.