Effect Of Temperature On Rate Of Reaction Arrhenius Equation With Faq S

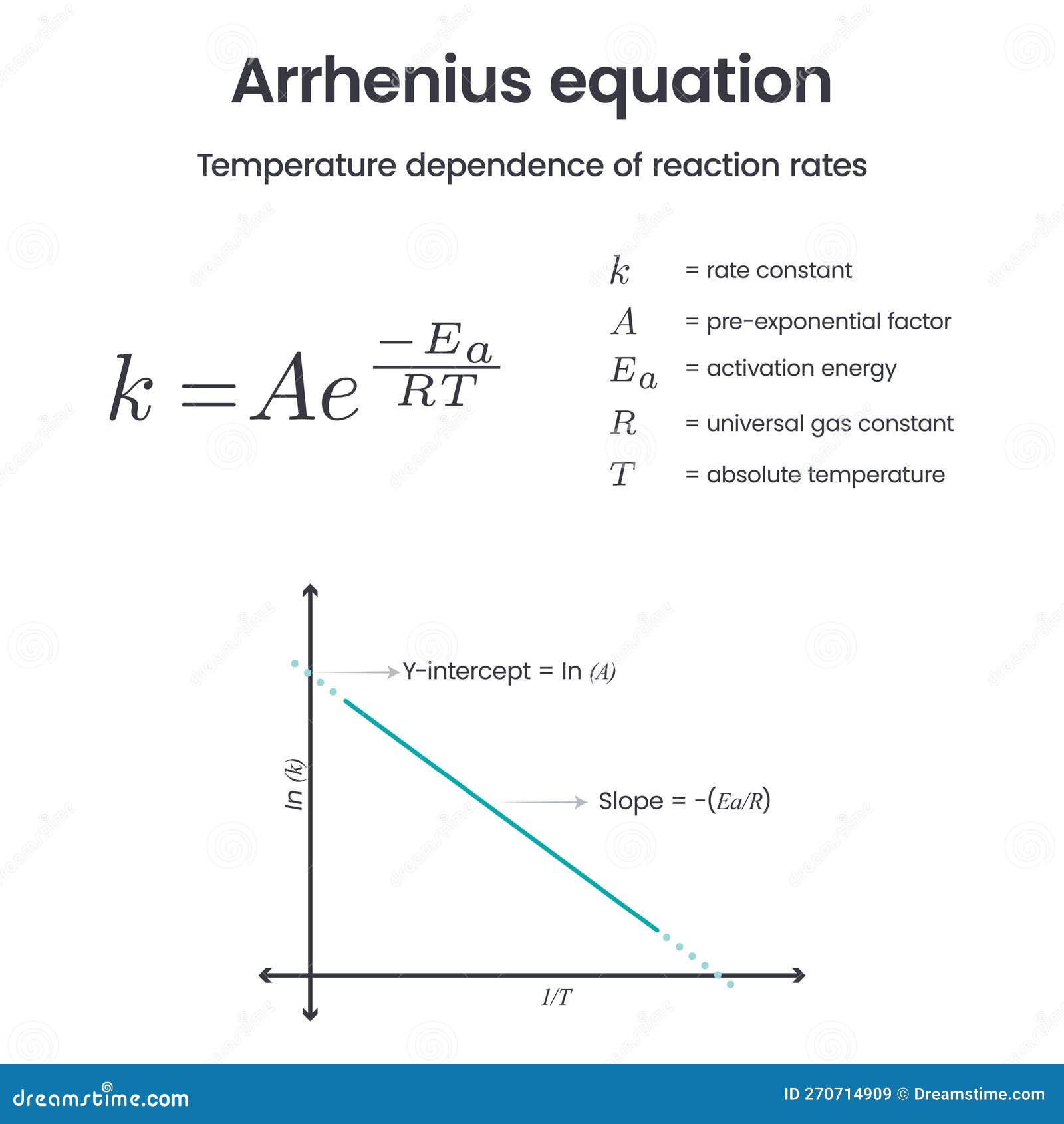
Effect Of Temperature On Rate Of Reaction Arrhenius Equation With Faq S In 1889, svante arrhenius extended the work of j.h van’t hoff and proposed an equation that related temperature and the rate constant for a reaction quantitatively. the proposed equation was named as arrhenius equation. arrhenius equation. here is the arrhenius equation which shows the temperature dependence of the rate of a chemical reaction. Equation 14.9.3 14.9.3 is known as the arrhenius equation and summarizes the collision model of chemical kinetics, where t t is the absolute temperature (in k) and r is the ideal gas constant [8.314 j (k·mol)]. ea e a indicates the sensitivity of the reaction to changes in temperature.
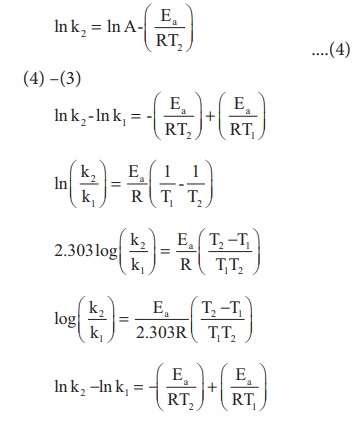
Arrhenius Equation The Effect Of Temperature On Reaction Rate The arrhenius equation, relates the activation energy and the rate constant, k, for many chemical reactions. in this equation, r is the ideal gas constant, which has a value 8.314 j mol·k, t is the temperature in kelvin, e a is the activation energy in joules per mole, e is the constant 2.7183, and a is a constant called the frequency factor, which is related to the frequency of collisions. In such cases, the reaction is nearly instantaneous. the arrhenius equation (equation 1.8.1) describes quantitatively much of what we have already discussed about reaction rates. for two reactions at the same temperature, the reaction with the higher activation energy has the lower rate constant and the slower rate. The arrhenius equation, arrhenius definition of an acid, lunar crater arrhenius, the mountain of arrheniusfjellet and the arrhenius labs at stockholm university are named after him. today, arrhenius is best known for his study published in 1896, on the greenhouse effect. This video aimed at a level students, explains why temperature affects the reaction rate, in terms of collision theory and shows them how to calculate the ef.
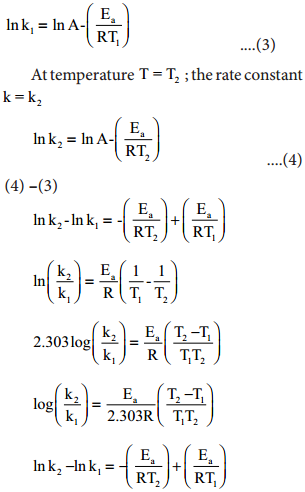
Arrhenius Equation вђ The Effect Of Temperature On Reaction Rate The arrhenius equation, arrhenius definition of an acid, lunar crater arrhenius, the mountain of arrheniusfjellet and the arrhenius labs at stockholm university are named after him. today, arrhenius is best known for his study published in 1896, on the greenhouse effect. This video aimed at a level students, explains why temperature affects the reaction rate, in terms of collision theory and shows them how to calculate the ef. Using the arrhenius equation. the effect of a change of temperature. you can use the arrhenius equation to show the effect of a change of temperature on the rate constant and therefore on the rate of the reaction. if the rate constant doubles, for example, so also will the rate of the reaction. The fraction of the colliding molecules that have an energy greater than or equal to ea. temperature dependence of the rate constant: increasing the temperature of a reaction generally speeds up the process (increases the rate) because the rate constant increases according to the arrhenius equation. rate (m s 1) = k [a]x[b]y.

Arrhenius Equation Increasing Temperature Increases The Rate Reaction Using the arrhenius equation. the effect of a change of temperature. you can use the arrhenius equation to show the effect of a change of temperature on the rate constant and therefore on the rate of the reaction. if the rate constant doubles, for example, so also will the rate of the reaction. The fraction of the colliding molecules that have an energy greater than or equal to ea. temperature dependence of the rate constant: increasing the temperature of a reaction generally speeds up the process (increases the rate) because the rate constant increases according to the arrhenius equation. rate (m s 1) = k [a]x[b]y.
Arrhenius Equation Physical Chemistry Science Vector Infographic Stock

Comments are closed.