Draw The Additional Resonance Structures Of The Structure Below Include All Valence Lone Pairs In

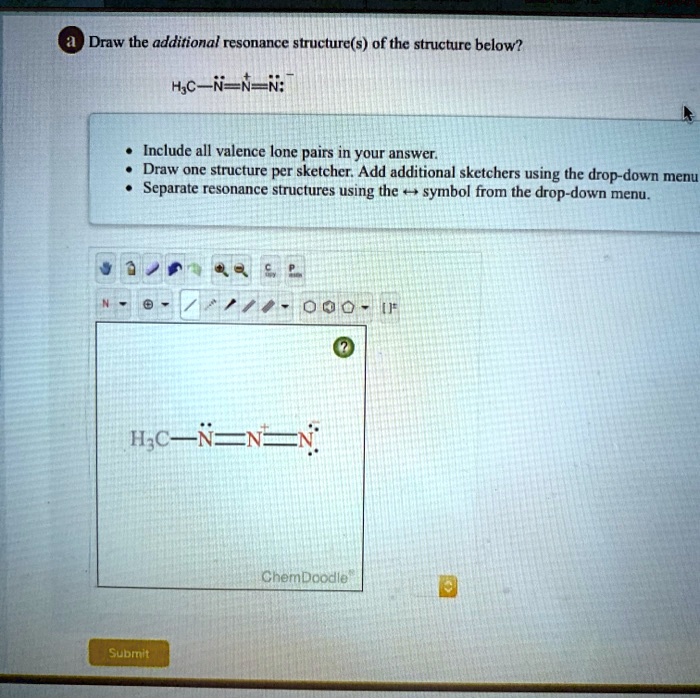
Oneclass Draw The Additional Resonance Structure S Of The Structure Question: draw the additional resonance structure (s) of the structure below? h2c=n=n: • include all valence lone pairs in your answer. • draw one structure per sketcher. add additional sketchers using the drop down menu in the bottom right corner. . separate resonance structures using the symbol from the drop down menu. there are 2 steps. The general approach is described below: draw the lewis structure & resonance for the molecule (using solid lines for bonds). where there can be a double or triple bond, draw a dotted line ( ) for the bond. draw only the lone pairs found in all resonance structures, do not include the lone pairs that are not on all of the resonance structures.

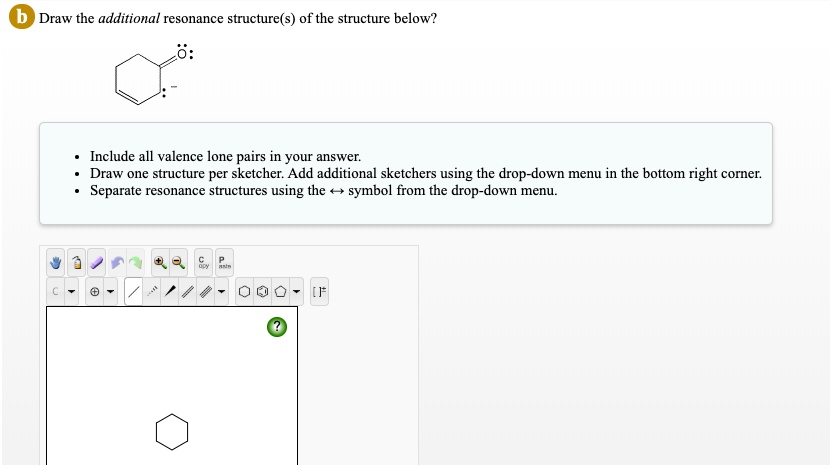
Solved Draw The Additional Resonance Structure S Of The Structure Exercise 2.4.5 2.4. 5. below is a minor resonance contributor of a species known as an ‘enamine’, which we will study more in section 19.8 (formation of enamines) section 23.12 (reactions of enamines). draw the major resonance contributor for the enamine, and explain why your contributor is the major one. answer. Example 2.5.1 2.5. 1. draw the major resonance contributor of the structure below. include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. explain why your contributor is the major one. Step 1. calculate the total number of valence electrons from each atom. carbon atom = 4. oxygen atoms (3*6) = 18. for ( 2) charge = 2. ** consider the 2 charge at the last step (i.e. this molecule has two extra electrons). step 2. when there is more than one type of atom, keep the least electronegative or metallic atom as the central atom. Question: draw the additional resonance structure (s) of the structure below? . include all valence lone pairs in your answer. . draw one structure per sketcher. add additional sketchers using the dropdown menu in the bottom nght corner separate resonance structures using the t symbol from the dropdown menu. . c p.
Solved Draw The Additional Resonance Structure S Of The Structure Step 1. calculate the total number of valence electrons from each atom. carbon atom = 4. oxygen atoms (3*6) = 18. for ( 2) charge = 2. ** consider the 2 charge at the last step (i.e. this molecule has two extra electrons). step 2. when there is more than one type of atom, keep the least electronegative or metallic atom as the central atom. Question: draw the additional resonance structure (s) of the structure below? . include all valence lone pairs in your answer. . draw one structure per sketcher. add additional sketchers using the dropdown menu in the bottom nght corner separate resonance structures using the t symbol from the dropdown menu. . c p. Solution. step 1: draw the lewis structure & resonance. step 2: combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. step 3: add only the lone pairs found on all resonance structures. the bottom is the finished resonance hybrid for co32 . Step 1: draw the lewis structure & resonance. step 2: combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. step 3: add only the lone pairs found on all resonance structures. the bottom is the finished resonance hybrid for co32 .
Solved Draw The Additional Resonance Structure S Of The Structure Solution. step 1: draw the lewis structure & resonance. step 2: combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. step 3: add only the lone pairs found on all resonance structures. the bottom is the finished resonance hybrid for co32 . Step 1: draw the lewis structure & resonance. step 2: combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. step 3: add only the lone pairs found on all resonance structures. the bottom is the finished resonance hybrid for co32 .
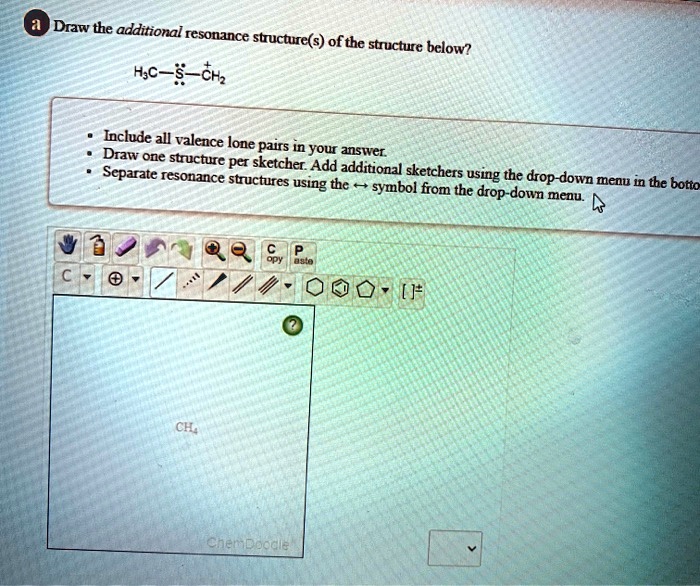
Solved Draw The Additional Resonance Structure S Ofthe Structure

Comments are closed.