Difference Between Physical And Chemical Change Definition

Difference Between Physical And Chemical Change Definition Examples Selected text level. matter is capable of undergoing changes, which are classified as either physical or chemical. physical changes in matter are often reversible: an ice cube can melt into liquid water, and then the liquid water can be frozen back into an ice cube. chemical changes, on the other hand, are not reversible: a log burned in a fire. Physical changes. a physical change is a change in matter that alters its form but not its chemical identity. the size or shape of matter often changes, but there is no chemical reaction. phase changes are physical changes. these include melting, boiling, vaporization, freezing, sublimation and deposition. breaking, crumpling, or molding matter.

Physical Vs Chemical Changes Worksheet Physical change is a temporary change. a chemical change is a permanent change. a physical change affects only physical properties i.e. shape, size, etc. chemical change both physical and chemical properties of the substance including its composition. a physical change involves very little to no absorption of energy. Chemical change vs. physical change. the difference between a physical reaction and a chemical reaction is composition. in a chemical reaction, there is a change in the composition of the substances in question; in a physical change there is a difference in the appearance, smell, or simple display of a sample of matter without a change in. Color changes indicate chemical change. the following reaction occurs: fe o2 → fe2o3 fe o 2 → fe 2 o 3. physical: because none of the properties changed, this is a physical change. the green mixture is still green and the colorless solution is still colorless. they have just been spread together. Physical changes involve the changes in the arrangement of atoms. chemical changes involve changes in the chemical composition of the substance. energy. physical changes absorb or release a comparatively less amount of energy. chemical changes are accompanied by the absorption or release of a significant amount of energy.
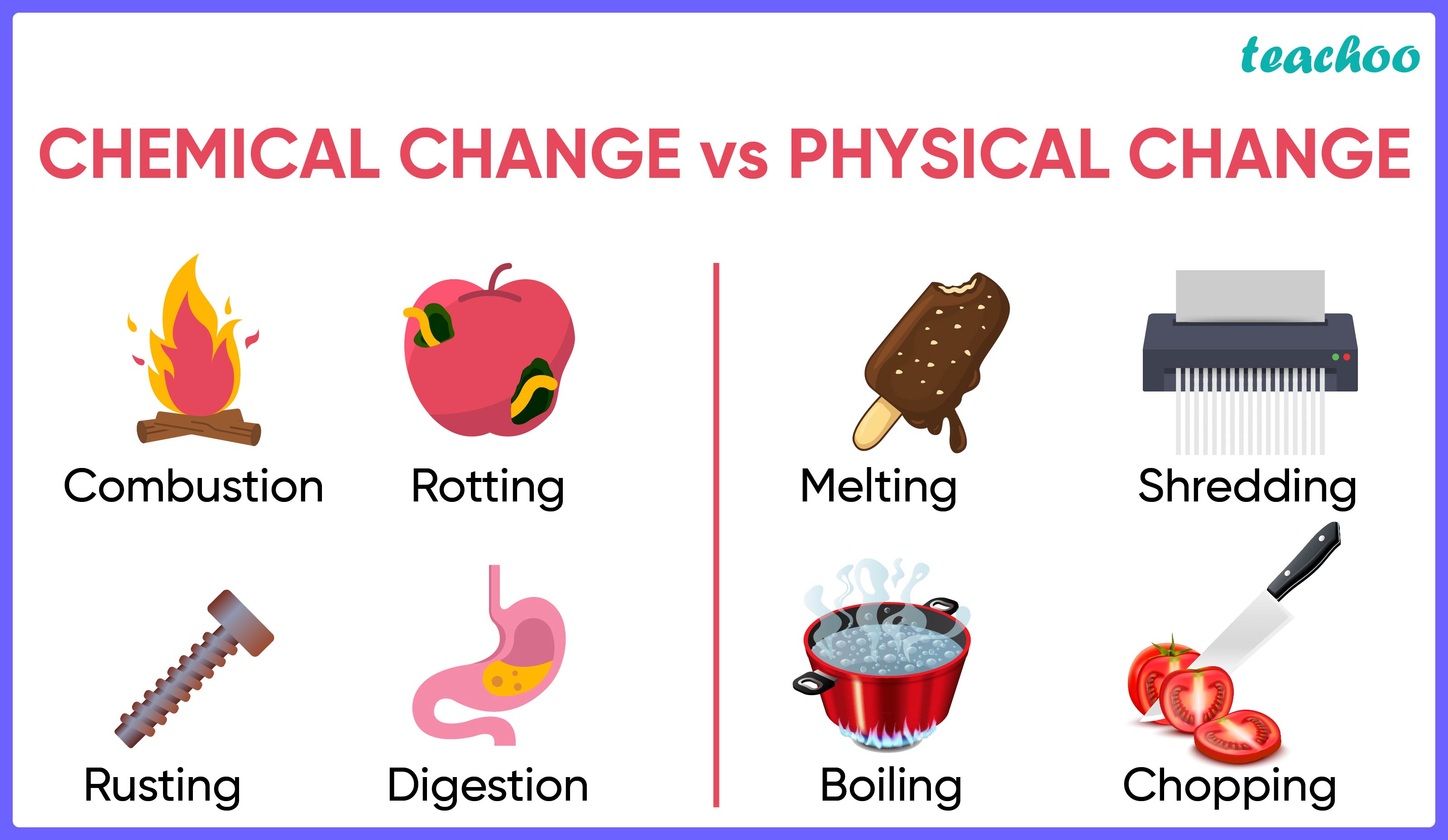
Difference Between Physical Change And Chemical Changes In Table Color changes indicate chemical change. the following reaction occurs: fe o2 → fe2o3 fe o 2 → fe 2 o 3. physical: because none of the properties changed, this is a physical change. the green mixture is still green and the colorless solution is still colorless. they have just been spread together. Physical changes involve the changes in the arrangement of atoms. chemical changes involve changes in the chemical composition of the substance. energy. physical changes absorb or release a comparatively less amount of energy. chemical changes are accompanied by the absorption or release of a significant amount of energy. Differences from physical changes. formation of new substances: the hallmark of chemical changes is the creation of entirely new substances, distinguishing them from physical changes. irreversibility: chemical changes are generally irreversible, setting them apart from the reversible nature of physical changes. Grouped together, they form molecules. matter can go through changes. some changes to matter are physical. other changes are chemical. the two kinds of changes are very different from each other. physical changes can often be reversed. for example, think of an ice cube in a hot room. the ice will melt and become water.

Comments are closed.