Difference Between Melting Point And Freezing Point Compare The

Difference Between Melting Point And Freezing Point Compare The One key difference between freezing point and melting point is the temperature range at which they occur. the freezing point is typically lower than the melting point for most substances. this is because the transition from a liquid to a solid requires the removal of heat energy, which lowers the temperature. The melting point of a solid is the temperature at which the vapor pressure of the liquid phase and the solid phase are equal and at equilibrium. if you increase the temperature, the solid will melt. if you decrease the temperature of a liquid past the same temperature, it may or may not freeze! this is supercooling and it occurs with many.

Melting Vs Freezing Point Difference And Comparison 1.the melting point is the temperature at which a solid is changed into a liquid by applying heat and pressure while the freezing point is the temperature at which a liquid is changed into a solid. 2.while most substances, especially pure substances, have the same melting and freezing points. some substances, like mixtures and compounds, have. Melting point and freezing point refer to temperatures at which substances change states between solid and liquid, but they represent these changes in opposite directions. while the melting point is the temperature at which a solid becomes a liquid, indicating the end of solidity, the freezing point marks the temperature at which a liquid solidifies, signifying the loss of fluidity. Key differences. melting point is defined as the temperature at which a solid substance transitions to its liquid state. in contrast, freezing point is the temperature at which a liquid substance turns into a solid. both represent a phase change, but in opposite directions. melting point is a critical property for materials like metals and. Key differences. the melting point is the temperature at which a solid turns into a liquid. in contrast, the freezing point is the temperature at which a liquid solidifies into a solid. 14. during the melting point, energy is absorbed by the solid to overcome molecular bonds. at the freezing point, the liquid releases energy as it forms.

Difference Between Melting Point And Freezing Point Compare The Key differences. melting point is defined as the temperature at which a solid substance transitions to its liquid state. in contrast, freezing point is the temperature at which a liquid substance turns into a solid. both represent a phase change, but in opposite directions. melting point is a critical property for materials like metals and. Key differences. the melting point is the temperature at which a solid turns into a liquid. in contrast, the freezing point is the temperature at which a liquid solidifies into a solid. 14. during the melting point, energy is absorbed by the solid to overcome molecular bonds. at the freezing point, the liquid releases energy as it forms. The melting point of solid oxygen, for example, is 218.4 o c. liquids have a characteristic temperature at which they turn into solids, known as their freezing point. in theory, the melting point of a solid should be the same as the freezing point of the liquid. in practice, small differences between these quantities can be observed. Main differences between melting and freezing points. the melting point occurs when the substance changes its state and turns solid to liquid, but the freezing point occurs when the meaning changes and gets converted from liquid to solid. atmospheric pressure is a significant factor in melting point, but external pressure is a major factor in.
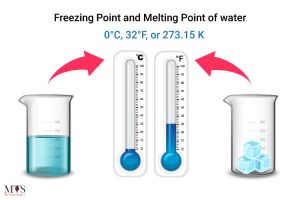
Water Freezing Point вђ Definition Factors Affecting It Supercooled The melting point of solid oxygen, for example, is 218.4 o c. liquids have a characteristic temperature at which they turn into solids, known as their freezing point. in theory, the melting point of a solid should be the same as the freezing point of the liquid. in practice, small differences between these quantities can be observed. Main differences between melting and freezing points. the melting point occurs when the substance changes its state and turns solid to liquid, but the freezing point occurs when the meaning changes and gets converted from liquid to solid. atmospheric pressure is a significant factor in melting point, but external pressure is a major factor in.

юааmeltingюаб юааpointюаб юааvsюаб юааfreezingюаб юааpointюаб таф Whatтащs The юааdifferenceюаб

Comments are closed.