Designing And Engineering Of Pharmaceutical Water Systems

Pharmaceutical Water Systems For Purified Water Iwater 3.1 basic water system monitoring requirements 5 3.2 purified water (pw) usp37 – nf32 6 3.3 water for injection (wfi) – usp37 – nf32 6 4. biofilm 7 5. designing and engineering pharmaceutical 7 water systems 5.1 pre treatment 7 5.2 purification 8 5.3 storage and distribution 9 6. instrumentation recommendations for 10. This course has been substantially updated to feature the guiding principles of the ispe baseline guide: water and steam systems (second edition) with particular emphasis placed upon microbial control and laboratory water as well as key design philosophies. the principles of design and operation of water systems used directly in pharmaceutical manufacturing and laboratory applications.

Designing And Engineering Of Pharmaceutical Water Systems Background. water is one of the most important raw materials in the manufacture of pharmaceutical products. in order to produce water of an appropriate quality, water systems have to fulfil considerable requirements, which are partly set out in detail in the relevant pharmaceutical regulations. although the characteristics of pharmaceu tical. Beginning with a brief overview of the theory and application of the technology, william collentro clarifies the seemingly overwhelming engineering aspects as he discusses design considerations, operation, maintenance, validation, and regulatory related topics bases upon personal experience with more than 400 pharmaceutical and related water. Abstract. a major new work on all aspects of water, the most used raw material ingredient in the pharmaceutical and biotechnology industries used as an excipient in pharmaceutical formulations, as. A major new work on all aspects of water, the most used raw material ingredient in the pharmaceutical and biotechnology industries used as an excipient in pharmaceutical formulations, as a cleaning agent, and as a separately packaged product diluent.drawing on the author's extensive field experience with more than 400 pharmaceutical and related.
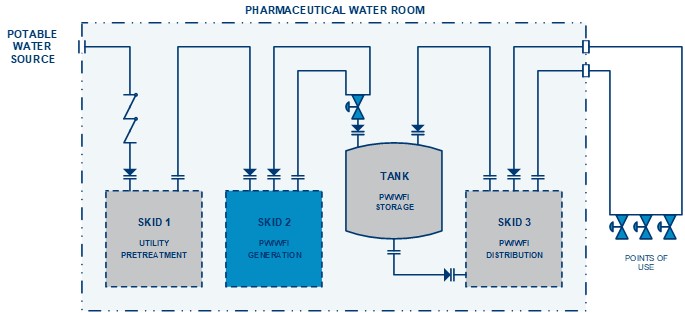
Designing And Engineering Of Pharmaceutical Water Systems 47 Off Abstract. a major new work on all aspects of water, the most used raw material ingredient in the pharmaceutical and biotechnology industries used as an excipient in pharmaceutical formulations, as. A major new work on all aspects of water, the most used raw material ingredient in the pharmaceutical and biotechnology industries used as an excipient in pharmaceutical formulations, as a cleaning agent, and as a separately packaged product diluent.drawing on the author's extensive field experience with more than 400 pharmaceutical and related. This chapter deals primarily with usp purified water and the most stringent type of produced water, water for injection (wfi). it focuses primarily on heat transfer operations. by observation, the difference between heat transfer and thermodynamics for project and process tasks is the time factor. Pharma water generation usp wfi & purified water training course (t04) this course will cover the principles of design and operation of water systems used directly in pharmaceutical manufacturing and laboratory applications, including the essential concepts and principles of systems used to generate usp and non compendial waters. these concepts.

Pdf Compliance Design Pharmaceutical Water Systemsв в в Designing And This chapter deals primarily with usp purified water and the most stringent type of produced water, water for injection (wfi). it focuses primarily on heat transfer operations. by observation, the difference between heat transfer and thermodynamics for project and process tasks is the time factor. Pharma water generation usp wfi & purified water training course (t04) this course will cover the principles of design and operation of water systems used directly in pharmaceutical manufacturing and laboratory applications, including the essential concepts and principles of systems used to generate usp and non compendial waters. these concepts.

Designing And Engineering Of Pharmaceutical Water Systems

Comments are closed.