Decantation Process Of Separating Mixtures

Decantation Mixtures Definition Examples Applications Decantation definition. decantation is the process of separation of liquid from solid and other immiscible (non mixing) liquids, by removing the liquid layer at the top from the layer of solid or liquid below. the process can be carried out by tilting the mixture after pouring out the top layer. this process can also be used to separate two. Decantation is a process that separates components of a mixture based on differences in density. you may encounter decantation in everyday life with wine or spirits, but it’s also a powerful technique in chemistry for separating a solid from a liquid or isolating two immiscible liquids. decantation is easy, but one disadvantage is that it.

What Is Decantation Definition And Examples Chemistry Decanting a liquid from a solid. decantation is a process for the separation of mixtures of immiscible liquids or of a liquid and a solid mixture such as a suspension. [1] the layer closer to the top of the container—the less dense of the two liquids, or the liquid from which the precipitate or sediment has settled out—is poured off. Decantation is a process to separate mixtures by removing a liquid layer that is free of a precipitate, or the solids deposited from a solution. the purpose may be to obtain a decant (liquid free from particulates) or to recover the precipitate. decantation relies on gravity to pull precipitate out of the solution, so there is always some loss. Evaporation is a technique used to separate out homogeneous mixtures that contain one or more dissolved salts. the method drives off the liquid components from the solid components. the process typically involves heating the mixture until no more liquid remains. prior to using this method, the mixture should only contain one liquid component. Decanting is also a chemical laboratory process used to separate mixtures. in its simplest form, it just means allowing a mixture of solid and liquid or two immiscible liquids to settle and separate by gravity. this process can be slow and tedious without the aid of a centrifuge. once the mixture components have separated, the lighter liquid is.

Decantation Mixtures Definition Examples Applications Evaporation is a technique used to separate out homogeneous mixtures that contain one or more dissolved salts. the method drives off the liquid components from the solid components. the process typically involves heating the mixture until no more liquid remains. prior to using this method, the mixture should only contain one liquid component. Decanting is also a chemical laboratory process used to separate mixtures. in its simplest form, it just means allowing a mixture of solid and liquid or two immiscible liquids to settle and separate by gravity. this process can be slow and tedious without the aid of a centrifuge. once the mixture components have separated, the lighter liquid is. Decantation is a separation process that allows for two immiscible liquids, or a liquid and solid, to be separated if a suitable amount of time is given for the substances to settle. based on the. Figure 1.67: a) sodium sulfate sticking to the glassware, b) decanting a solid liquid mixture, c) using a glass stirring rod during decanting. when there is a need to separate a solid liquid mixture, on occasion it is possible to pour off the liquid while leaving the solid behind. this process is called decanting, and is the simplest ….
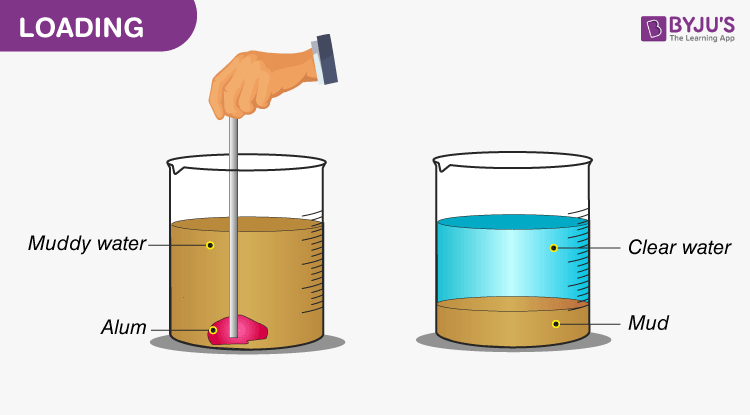
Decantation Mixtures Definition Examples Applications Decantation is a separation process that allows for two immiscible liquids, or a liquid and solid, to be separated if a suitable amount of time is given for the substances to settle. based on the. Figure 1.67: a) sodium sulfate sticking to the glassware, b) decanting a solid liquid mixture, c) using a glass stirring rod during decanting. when there is a need to separate a solid liquid mixture, on occasion it is possible to pour off the liquid while leaving the solid behind. this process is called decanting, and is the simplest ….
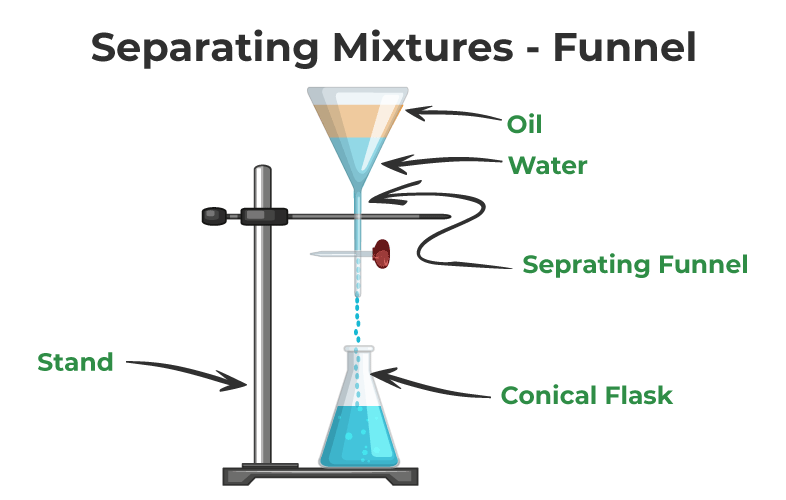
Decantation Process Of Separating Mixtures

Comments are closed.