Convert Mole To Atoms Or Atoms To Mole

Moles To Atoms Conversion Chemistry Youtube Conversion of moles to atoms. the mole is the unit of measurement for amount of substance in the international system of units (si). it is defined as exactly 6.02214076×10 23 particles, which may be molecules, atoms, ions or electrons, depending on the nature of the substance. the number 6.02214076×10 23 is known as the avogadro’s number. Atoms to moles formula. using avogadro’s constant, the formula to convert atoms to moles is: mol = atoms ÷ 6.02214076 × 10 23. thus, the amount of a purse substance in moles is equal to the number of atoms divided by avogadro’s constant, or 6.02214076 × 10 23.

How To Convert Atoms To Mole Moles To Atom Quick Conversation So, to find the number of hydrogen atoms in a mole of water molecules, the problem can be solved using conversion factors: 1mol h2o × 6.02 ×1023 moleculesh2o 1 molh2o × 2atoms h 1 moleculeh2o = 1.20 ×1024 atomsh 1 mol h 2 o × 6.02 × 10 23 molecules h 2 o 1 mol h 2 o × 2 atoms h 1 molecule h 2 o = 1.20 × 10 24 atoms h. The value of the moles is equal to the number of atoms of 12 g c 12 carbon atoms= 6.022140857 x 10^23 atoms. we take c 12 as a standard of measuring avogadro's number. the moles to atoms formula: 1 mole= 6.0221415e 23 atoms. the moles to atoms conversion and atoms to moles conversion are interchangeable to each other. Number of atoms = number of moles x avogadro’s number. number of atoms = 2 moles x 6.02214076 × 10 23 atoms mole ≈ 1.2044 × 10 24 atoms. another way of calculating the number of atoms is through a two step process: moles to grams and then grams to atoms. this method requires the atomic mass of an element, which you get from the periodic. How to work atoms to moles calculator. user input: the user provides the number of atoms of a particular element or compound. avogadro's number: the calculator uses avogadro's number, which is approximately \(6.022 \times 10^{23}\) entities (atoms, molecules, ions) per mole.

Converting From Atoms To Moles Youtube Number of atoms = number of moles x avogadro’s number. number of atoms = 2 moles x 6.02214076 × 10 23 atoms mole ≈ 1.2044 × 10 24 atoms. another way of calculating the number of atoms is through a two step process: moles to grams and then grams to atoms. this method requires the atomic mass of an element, which you get from the periodic. How to work atoms to moles calculator. user input: the user provides the number of atoms of a particular element or compound. avogadro's number: the calculator uses avogadro's number, which is approximately \(6.022 \times 10^{23}\) entities (atoms, molecules, ions) per mole. This chemistry video explains the conversion process of moles to atoms and how to convert the number of atoms to moles. this tutorial is useful for calculat. Therefore, given the relationship 1 mol = 6.022 x 10 23 atoms, converting between moles and atoms of a substance becomes a simple dimensional analysis problem. converting moles to atoms given a known number of moles (x), one can find the number of atoms (y) in this molar quantity by multiplying it by avogadro's number:.
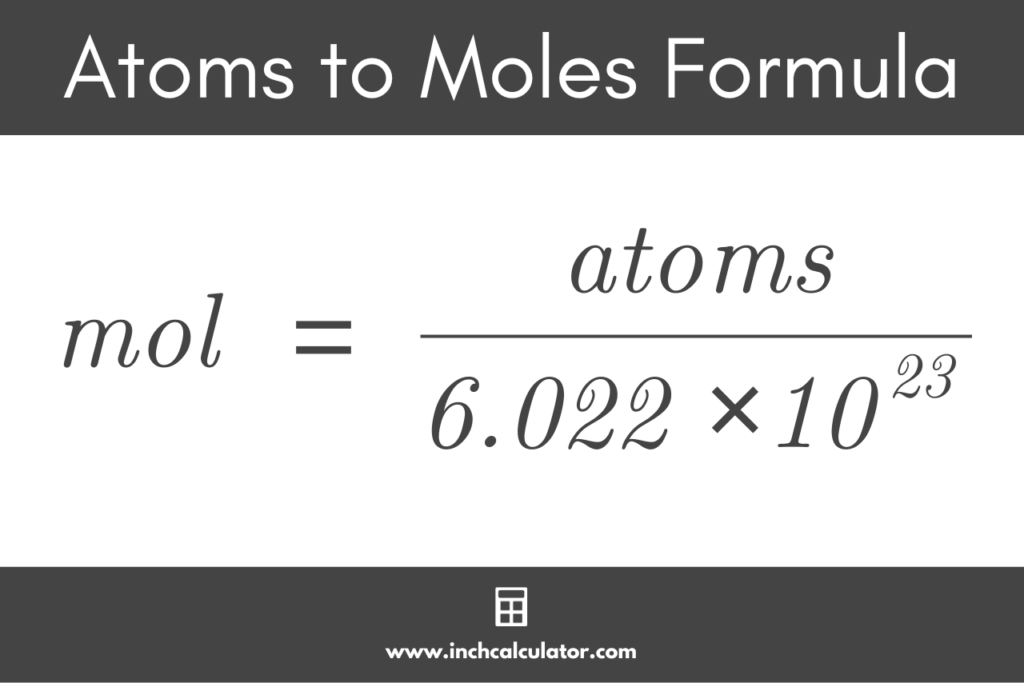
Atoms To Moles Calculator Inch Calculator This chemistry video explains the conversion process of moles to atoms and how to convert the number of atoms to moles. this tutorial is useful for calculat. Therefore, given the relationship 1 mol = 6.022 x 10 23 atoms, converting between moles and atoms of a substance becomes a simple dimensional analysis problem. converting moles to atoms given a known number of moles (x), one can find the number of atoms (y) in this molar quantity by multiplying it by avogadro's number:.

Moles Converting Between Moles Atoms And Molecules Bartlett High School

Comments are closed.