Concentration Mole Fraction

Concentration Mole Fraction Mole fraction is numerically identical to the number fraction, which is defined as the number of particles (molecules) of a constituent ni divided by the total number of all molecules ntot. whereas mole fraction is a ratio of amounts to amounts (in units of moles per moles), molar concentration is a quotient of amount to volume (in units of. The mole fraction; the molarity; the molality; given: volume percent and density. asked for: mass percentage, mole fraction, molarity, and molality. strategy: use the density of the solute to calculate the mass of the solute in 100.0 ml of solution. calculate the mass of water in 100.0 ml of solution.
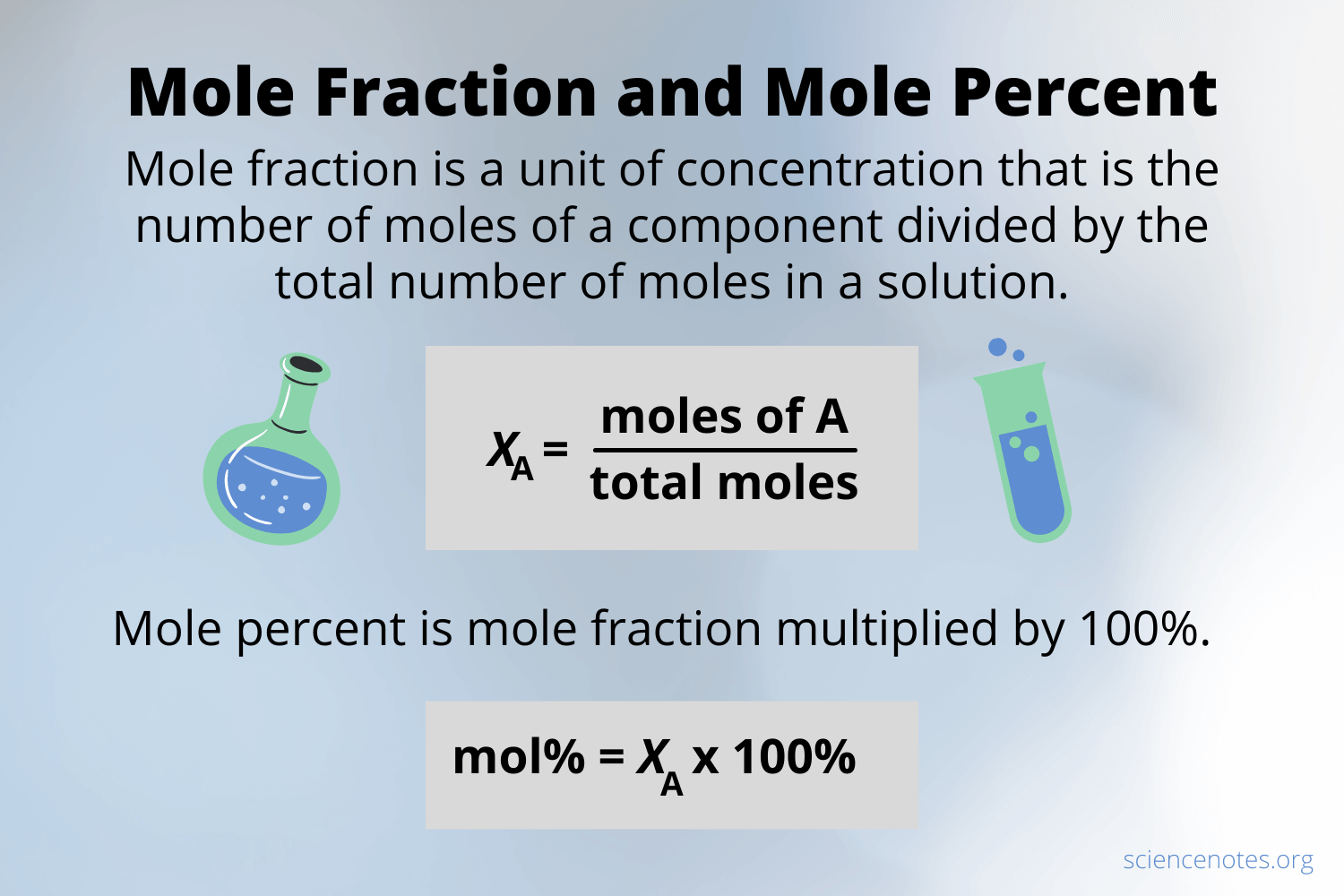
Mole Fraction Study Guide Inspirit Learning Inc Molar concentration (also called molarity, amount concentration or substance concentration) the conversion to mole fraction is given by = ¯, where ¯ is the. The moles of solute present in the solution. the mass of solvent (in kilograms) in the solution. to calculate molality we use the equation: top mole fraction. the mole fraction, x, of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components in the solution. In chemistry, the mole fraction is a unit of concentration that is the number of moles of a component divided by the total number of moles of a solution or mixture. the mole fraction is a dimensionless number. the sum of all of the mole fractions equals 1. the symbol for mole fraction is the capital letter x or the lowercase greek letter chi. 14.12: mole fraction. sulfur dioxide is a by product of many processes, both natural and human made. massive amounts of this gas are released during volcanic eruptions. humans produce sulfur dioxide by burning coal. when in the atmosphere, the gas has a cooling effect by reflecting sunlight away from the earth.
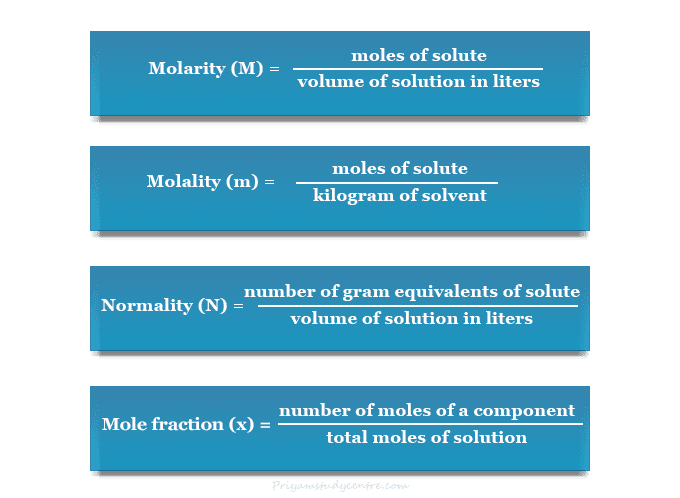
Concentration Calculation Formula Chemistry In chemistry, the mole fraction is a unit of concentration that is the number of moles of a component divided by the total number of moles of a solution or mixture. the mole fraction is a dimensionless number. the sum of all of the mole fractions equals 1. the symbol for mole fraction is the capital letter x or the lowercase greek letter chi. 14.12: mole fraction. sulfur dioxide is a by product of many processes, both natural and human made. massive amounts of this gas are released during volcanic eruptions. humans produce sulfur dioxide by burning coal. when in the atmosphere, the gas has a cooling effect by reflecting sunlight away from the earth. Concentration units based on moles. mole fraction: the mole fraction of a substance is the fraction of all of its molecules (or atoms) out of the total number of molecules (or atoms). it can also come in handy sometimes when dealing with the \(pv=nrt\) equation. \[\chi a= \dfrac{\text{number of moles of substance a}}{\text{total number of moles. Updated on december 01, 2019. mole fraction is a unit of concentration, defined to be equal to the number of moles of a component divided by the total number of moles of a solution. because it is a ratio, mole fraction is a unitless expression. the mole fraction of all components of a solution, when added together, will equal 1.

Ppt Solution Concentration Mass Percent Molality And Mole Fraction Concentration units based on moles. mole fraction: the mole fraction of a substance is the fraction of all of its molecules (or atoms) out of the total number of molecules (or atoms). it can also come in handy sometimes when dealing with the \(pv=nrt\) equation. \[\chi a= \dfrac{\text{number of moles of substance a}}{\text{total number of moles. Updated on december 01, 2019. mole fraction is a unit of concentration, defined to be equal to the number of moles of a component divided by the total number of moles of a solution. because it is a ratio, mole fraction is a unitless expression. the mole fraction of all components of a solution, when added together, will equal 1.

Comments are closed.