Column I Groups A N Me_3 B P Me_3 C Ome D
How To Read The Periodic Table Amnh Column i (groups) (a) n me 3 (b) p me 3 (c) ome (d) ch 3 column ii (effect) (p) i (q) i (r) r (s) r (t) hwatch the full video at: numerade . Key takeaways. in excel, select the columns you want to group. go to the data tab. open the outline drop down menu and pick "group." use the plus ( ) and minus ( ) buttons that appear to expand and collapse the group. if you organize a spreadsheet by columns, you may only want to work with specific columns at one time.

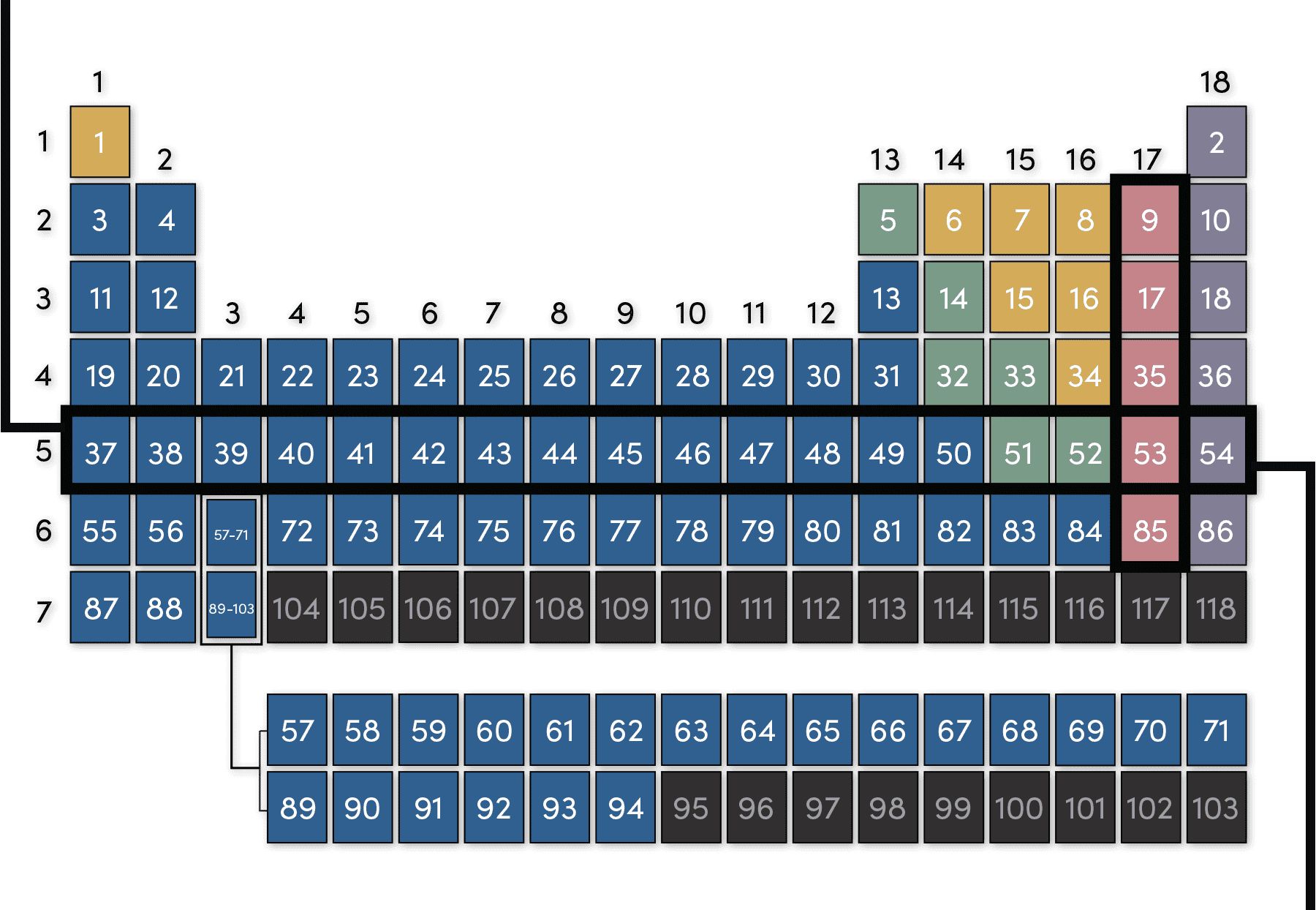
Periodic Table Groups And Periods A group is a vertical column down the periodic table, while a period is a horizontal row across the table. both groups and periods reflect the organization of electrons in atoms. element atomic number increases as you move down a group from top to bottom or across a period from left to right. an element group is a vertical column on the. Select the columns to be included in the inner group. on the data tab, in the outline group, click group. or press the shift alt right arrow shortcut. in our dataset, to group the q1 details, we select columns b through d and click group: in the same fashion, you can group q2 details (columns f through h). note. Transition metals: returning to the main body of the table, the remainder of groups 3 through 12 represent the rest of the transition metals. hard but malleable, shiny, and possessing good. The periodic table is organized like a big grid. each element is placed in a specific location because of its atomic structure. as with any grid, the periodic table has rows (left to right) and columns (up and down). each row and column has specific characteristics. for example, magnesium (mg) and calcium (mg) are found in column two and share.

Group N Vs Group A At Robin Graves Blog Transition metals: returning to the main body of the table, the remainder of groups 3 through 12 represent the rest of the transition metals. hard but malleable, shiny, and possessing good. The periodic table is organized like a big grid. each element is placed in a specific location because of its atomic structure. as with any grid, the periodic table has rows (left to right) and columns (up and down). each row and column has specific characteristics. for example, magnesium (mg) and calcium (mg) are found in column two and share. Another common method of categorization recognizes nine element families: alkali metals: group 1 (ia) one valence electron. alkaline earth metals: group 2 (iia) two valence electrons. transition metals: groups 3 12 two valence electrons. boron group or earth metals: group 13 (iiia) three valence electrons. Vocabulary. group (family): a vertical column in the periodic table. alkali metals: group 1a of the periodic table. alkaline earth metals: group 2a of the periodic table. halogens: group 7a of the periodic table. noble gases: group 8a of the periodic table. transition elements: groups 3 to 12 of the periodic table.

Comments are closed.