Co2 Molecular Geometry And Lewis Structure

Molecular Geometry Lewis Structure And Bond Angle Of Co2 For lewis structure of co2, you will now have two oxygen atoms forming double bonds with a carbon atom. as all the valence electrons of all the atoms are used, there are no lone pairs of electrons or non bonding pairs of electrons in the molecule. to further understand the molecular geometry of co2, let us quickly go through its hybridization. Co2 has a linear shape. the bond angle of co2 is 180°. the molecular geometry of any compound can be determined by the vsepr theory. the vsepr chart is attached below, which will give us an idea about this. so from the above chart, it’s clear that co2 is an ax2 type molecule, where x= bonded atom. thus the molecular geometry of the co2.

Co2 Lewis Structure And Molecular Geometry What S Insight Since the overall formal charge is zero, the above lewis structure of co 2 is most appropriate, reliable, and stable in nature. molecular geometry of co 2. co 2 molecular geometry is based on a linear arrangement. the presence of a sigma bond and valence electron pairs repelling each other force them to move to the opposite side of the carbon. This chemistry video explains how to draw the lewis structure of co2 also known as carbon dioxide. it also discusses the bond angle, molecular geometry, and. Co2 has a linear molecular geometry with a bond angle of 180° on a plan. molar mass of co2 is 44.01 g mol which is also known as molecular weight. carbon dioxide has an sp hybridization type because the steric number of central carbon is 2. carbon dioxide is a polar molecule but both c=o bonds are polar bonds. The molecular geometry of co2 is linear, with a bond angle of 180 degrees between the two oxygen atoms and the central carbon atom. what is the significance of the lewis structure for co2? the lewis structure for co2 helps us understand the bonding between the carbon and oxygen atoms and predict the molecule’s geometry, polarity, and reactivity.
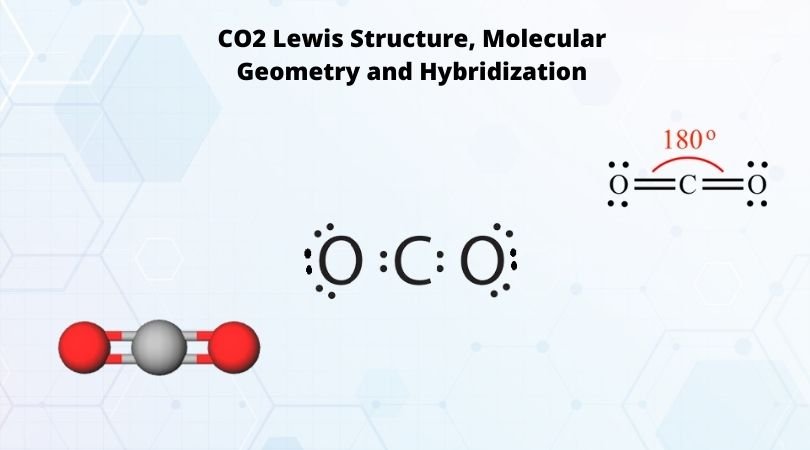
Co2 Lewis Structure Molecular Geometry And Hybridization Co2 has a linear molecular geometry with a bond angle of 180° on a plan. molar mass of co2 is 44.01 g mol which is also known as molecular weight. carbon dioxide has an sp hybridization type because the steric number of central carbon is 2. carbon dioxide is a polar molecule but both c=o bonds are polar bonds. The molecular geometry of co2 is linear, with a bond angle of 180 degrees between the two oxygen atoms and the central carbon atom. what is the significance of the lewis structure for co2? the lewis structure for co2 helps us understand the bonding between the carbon and oxygen atoms and predict the molecule’s geometry, polarity, and reactivity. I quickly take you through how to draw the lewis structure of co2 (carbon dioxide). i also go over hybridization, shape and bond angles. Co2 has a molecular geometry. according to the vsepr theory, the shape of a molecule can be predicted by considering the electron pair repulsion in the valence shell. from the lewis structure of co 2, there are two sigma bonds and no lone pair of electrons. the bonding pair of electrons around the central carbon atom will repel each other and.

Comments are closed.