Clinical Trial Recovery From Covid 19 Disruption
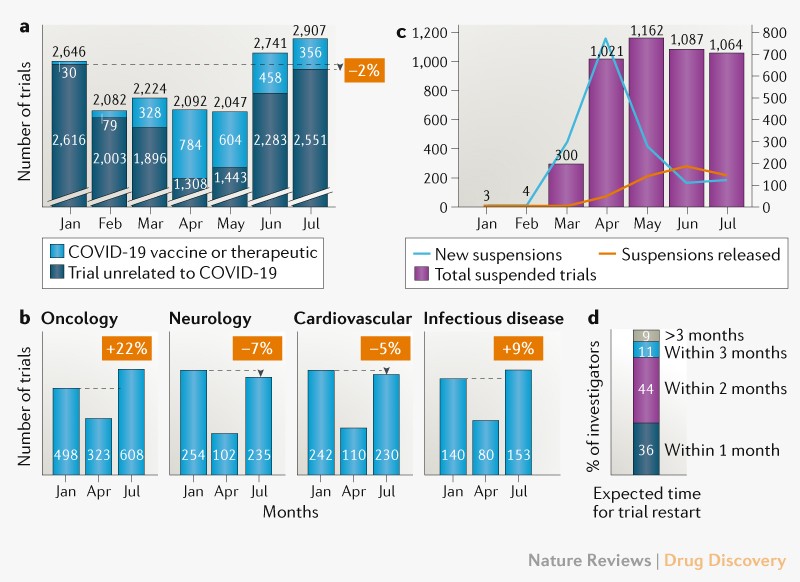
Clinical Trial Recovery From Covid 19 Disruption Clinical trial recovery from covid 19 disruption. since covid 19 emerged in january 2020, it has caused unprecedented disruption of clinical trials and ongoing patient care. around 1,000. Covid 19. clinical trials as topic* statistics & numerical data. coronavirus infections*. humans. pandemics*. pneumonia, viral*. clinical trial recovery from covid 19 disruption.
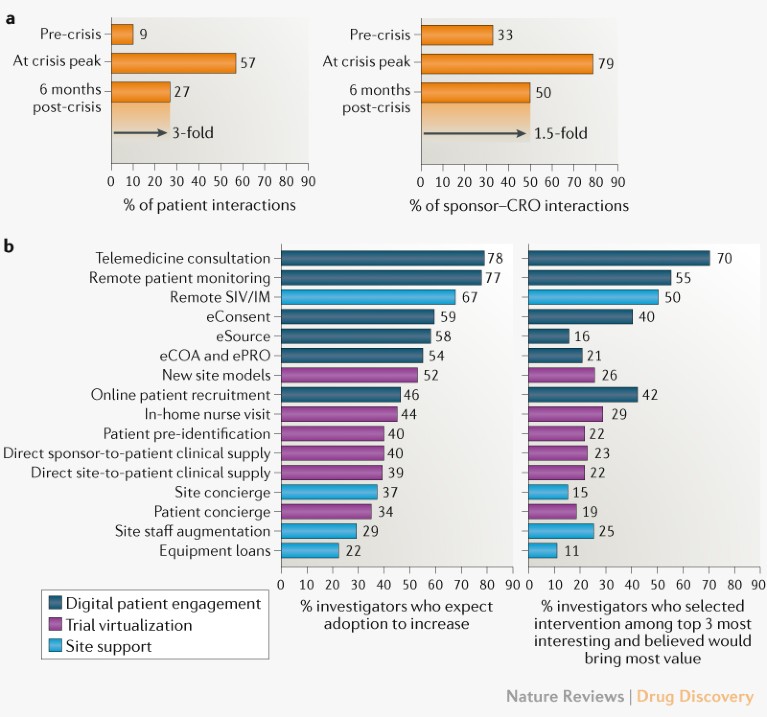
Clinical Trial Recovery From Covid 19 Disruption Title: clinical trial recovery from covid 19 disruption author: john z. xue subject: nature reviews drug discovery, doi:10.1038 d41573 020 00150 9. Th organization ictrp record of covid 19 trials. we used the study status field to identify suspended trials and searched for explicit mention o. reported reason for suspension.figure 1d and 2we conducted a double blinded, english language online survey of 245 clinical trial investigators and study coordinators, administered via third . To help understand the recovery from covid 19 disruption and the implications for the future conduct of clinical trials, data from clinicaltrials.gov is analysed and the ongoing challenges for between sponsors and contract research organizations (cros) taking place remotely are discussed. it has caused unprecedented disruption of clinical trials and ongoing patient care. around 1,000. Approximately 1 year ago, in march 2020, clinical trials were suddenly and severely disrupted by the covid 19 pandemic. pandemic related restrictions that limited or prevented in person visits resulted in unprecedented obstacles to clinical trial enrollment, data collection, and intervention delivery for many clinical trials. 1 as of april 21, 2021, clinicaltrials.gov listed 1773 suspended.

Use Of The Estimand Framework To Manage The Disruptive Effects Of Covid To help understand the recovery from covid 19 disruption and the implications for the future conduct of clinical trials, data from clinicaltrials.gov is analysed and the ongoing challenges for between sponsors and contract research organizations (cros) taking place remotely are discussed. it has caused unprecedented disruption of clinical trials and ongoing patient care. around 1,000. Approximately 1 year ago, in march 2020, clinical trials were suddenly and severely disrupted by the covid 19 pandemic. pandemic related restrictions that limited or prevented in person visits resulted in unprecedented obstacles to clinical trial enrollment, data collection, and intervention delivery for many clinical trials. 1 as of april 21, 2021, clinicaltrials.gov listed 1773 suspended. Covid 19 and readjusting clinical trials. the covid 19 pandemic has disrupted clinical trials worldwide, with long lasting effects on medical science. aaron van dorn reports. the covid 19 pandemic has created massive disruptions to clinical trial research across the world. as in other aspects of life, the virus has severely affected the ability. Recovery 1 year on: a rare success in the covid 19 clinical trial landscape. peter horby, co lead on the recovery platform trial, discusses the origins, lessons learned and future plans for one of.

Covid 19 And Cancer Trials Disruption And Recovery European Medical Covid 19 and readjusting clinical trials. the covid 19 pandemic has disrupted clinical trials worldwide, with long lasting effects on medical science. aaron van dorn reports. the covid 19 pandemic has created massive disruptions to clinical trial research across the world. as in other aspects of life, the virus has severely affected the ability. Recovery 1 year on: a rare success in the covid 19 clinical trial landscape. peter horby, co lead on the recovery platform trial, discusses the origins, lessons learned and future plans for one of.

Comments are closed.